For a printable PDF of this course and exam, click here.
Basics of Insulin Pump Therapy
Indications for using insulin pump therapy include one or more of the following1:
• Inability to achieve blood glucose (BG) goals with multiple daily injections of insulin: Pump delivery of insulin more closely mimics patients’ normal physiology.
• Recurrent hypo- or hyperglycemia: Pumps improve glycemic control by delivering an individualized basal rate of insulin supplemented with bolus doses to match patients’ carbohydrate (CHO) intake and correct any instances of hyperglycemia.
• Hypoglycemia unawareness: This condition is the absence of warning signs of low BG associated with long-term diabetes and/or the intensification of diabetes control and repeated episodes of hypoglycemia. By allowing individualized insulin doses to match patient requirements hour by hour, pumps are associated with fewer instances of severe hypoglycemia.
• Preconception and pregnancy: Pumps reduce the risk of severe hypoglycemia and can improve the management of morning sickness by eliminating the need to eat upon rising. (A correctly calculated basal rate maintains euglycemia, or normal BG.)
• Erratic work or activity schedule: Pumps allow freedom and flexibility in patients’ eating and activity schedules.
• Desire for flexibility: Pumps eliminate the frequency and inconvenience of multiple daily injections.
Insulin pump therapy isn’t without potential drawbacks, though. When considering whether patients are good candidates for this therapy, dietitians should weigh the following potential drawbacks against the possible benefits for each individual:
• Learning curve: Pump therapy requires education, skills, training, and initial intensive follow-up and management. CHO counting and matching insulin doses with CHO intake and basal needs are imperative. An understanding of pump therapy as well as realistic expectations about it and the ability to wear the pump, fill and replace the pump reservoirs, and perform the technical functions of the pump also are necessary.
• Frequent self-monitoring of BG: A minimum of four glucose checks daily (before meals and at bedtime) is essential, with additional checks as needed between meals, overnight, in relation to exercise, during times of illness and stress, and anytime BG becomes erratic.
• Possible weight gain: The freedom to eat without worrying about insulin injections may prompt some patients to indulge in high-calorie foods with little nutritional value that they had considered forbidden prior to pump therapy.
• Hypoglycemia: Inaccurate basal rate settings, miscalculation of CHO intake, overdosing a bolus delivery, or inadequate compensation for exercise can result in low BG, requiring prompt management.
• Unexpected hyperglycemia: Inaccurate basal rate settings, miscalculation of CHO intake, or underdosing bolus delivery can result in high BG. In addition, the rare pump failure or occasional infusion site occlusion, which prevents or delays insulin delivery, can result in high BG. Prompt identification and management of high BG is necessary to avoid ketoacidosis.
• Ketoacidosis: Pump malfunction and/or site occlusion may cause partial or total interruption of basal insulin delivery and because pumps use only rapid-acting insulin, there is no “background” insulin available to prevent high BG and the formation of ketones. Prompt identification and management of functional problems and high BG is imperative to avoid diabetic ketoacidosis.
• Skin irritation or infusion site infections: Site infections can occur from poor insertion techniques, lack of site rotation, or leaving the infusion set in one site for too long. Infusion set tape may cause redness, tenderness, itching, or rashes in those with sensitive skin. In addition, heavy perspiration or frequent participation in water sports may present problems with keeping the tape stuck to the skin.
• Cost: In addition to the price of the pump itself, there will be expenses for disposable supplies, including batteries, insulin reservoirs, infusion sets, and skin preparation products, that are necessary for operation. Some insurance companies cover all or some of these expenses, whereas others may provide for only the pump and not the supplies or vice versa.
Although this article doesn’t go into great detail on candidate selection, good prospects for insulin pump therapy should possess certain characteristics, including the following1:
• Motivation: The first few weeks of pump therapy require detailed record keeping, frequent BG checks, and regular communication with health care professionals.
• Realistic expectations: Patients must understand that pump therapy takes a lot of work on their part and won’t necessarily fix their BG problems.
• Ability to demonstrate independent diabetes management: The ability to demonstrate appropriate self-care behaviors provides the foundation for the advanced self-management skills required by pump users.
• Willingness to learn: CHO counting, premeal bolus dose calculations, and hypo- and hyperglycemia management are essential for pump use.
• Emotional stability: Behaviors that may interfere with pump therapy include untreated depression, eating disorders, manipulative behavior, and other psychoses.
When compared with multiple daily injections, studies have shown improved BG control in patients with type 1 and type 2 diabetes using insulin pump therapy.2,3 In a meta-analysis of 12 randomized controlled trials investigating type 1 diabetes and insulin pump therapy, the mean BG and the percentage of hemoglobin A1c was lower in those receiving pump therapy, equivalent to a difference in A1c of 0.51%.2
While there are fewer studies investigating pump therapy in type 2 diabetes patients, two studies have shown that pump therapy significantly improved glycemic control in most people with poorly controlled type 2 diabetes. One study compared insulin pump therapy with multiple daily injections, while the other study investigated multiple daily injections, oral antidiabetic agents, and basal insulin therapy compared with insulin pump therapy.3
Basic Functions of Insulin Pump Therapy
A normal functioning pancreas secretes insulin in two ways: basal and bolus. Basal insulin is the background release of insulin secreted continually to counteract increases in BG due to gluconeogenesis (the formation of glucose in the liver) or hormone fluctuations caused by stressors, activity, or metabolic changes. Bolus insulin is secreted periodically to counteract increases in BG following meals or to correct periodically high BG levels.
An insulin pump delivers rapid-acting insulin—aspart (NovoLog), lispro (Humalog), or glulisine (Apidra)—in such a way that it mimics both basal and bolus insulin needs. A basal rate profile is a series of basal rates, delivered in units per hour (u/hr), programmed into the pump to meet patients’ individual needs, resulting in a continuous 24-hour delivery of basal insulin. Basal rates can be adjusted every 30 minutes in increments as small as 0.05 u/hr.
Methods to determine an individual’s basal rate profile are based on total daily insulin needs and are outside the scope of this course. However, a sample 24-hour basal rate profile is shown in Figure 1.
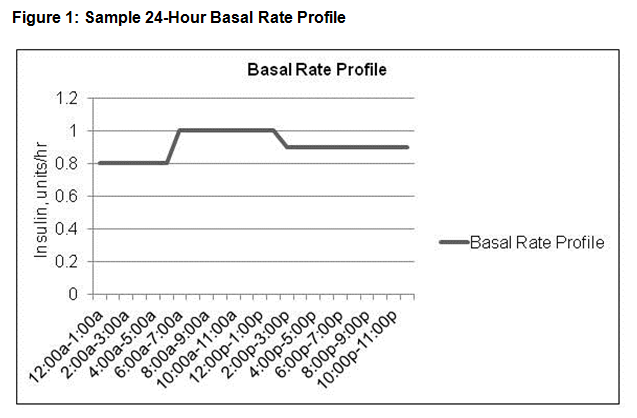
In this example, the individual receives insulin at the rate of 0.8 u/hr from midnight to 6 AM, 1 u/hr from 6 AM to 2 PM, and 0.9 u/hr from 2 PM to midnight; the total basal insulin dose is 21.8 u/day.
In response to CHO intake and/or elevated BG levels outside of the goal range, a patient programs the pump to deliver a bolus of insulin on demand. A meal bolus is the sum of the correction bolus and the CHO bolus. The correction bolus is the amount of insulin needed to bring BG to goal level, and the CHO bolus is the amount needed to counteract the rise in BG due to CHO grams consumed.
All modern insulin pumps have a bolus calculator that uses an individual’s sensitivity factor (SF) and insulin-to-carbohydrate ratio (ICR) to calculate the meal bolus of insulin. SF is the number of milligrams per deciliter (mg/dL) by which 1 unit of rapid-acting insulin will lower BG, while ICR is the grams of CHO counteracted by 1 unit of rapid-acting insulin.
Methods to calculate an individual’s ICR and SF are outside the scope of this course and were discussed in the continuing education course “Understanding Advanced Carbohydrate Counting” (available at http://www.TodaysDietitian.com/newarchives/120913p40.shtml). A sample meal bolus calculation can be found in Table 1.
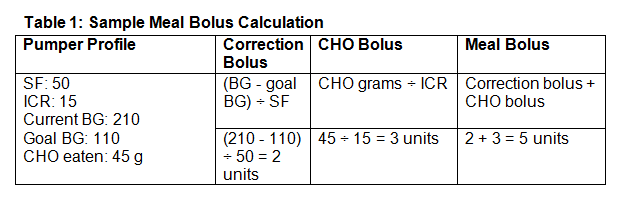
In this example, the patient’s SF is 50 (1 unit of rapid-acting insulin is needed for every 50 mg/dL that BG is over goal); the patient’s ICR is 15 (1 unit is needed for every 15 g of CHO); and the meal bolus calculated is 5 units.
Indications for Advanced Insulin Pump Therapy
Transient and recurrent changes in basal and bolus insulin needs occur from time to time. Day-to-day cycles, dawn phenomenon (an abnormal early-morning BG rise), acute illness, and stress can alter daily basal insulin needs. Activity also alters insulin needs, with moderate exercise increasing the risk of hypoglycemia during, shortly after, or for several hours after the activity, while strenuous exercise can lead to elevated BG.4
In addition to transient changes, prolonged recurring changes to basal needs (longer than 24 hours) can occur. Increased or decreased activity levels, menses, seasonal changes, and altered work schedules are examples of situations that can markedly affect insulin needs for prolonged periods of time.
The temporary basal rate feature on insulin pumps, discussed below, incrementally changes the amount of basal insulin delivered for a specified period of time to accommodate transient changes in basal insulin, such as during an exercise session or a sporting event, when basal needs may be less than usual. Also, an alternate basal rate profile can be developed and stored in the pump for use on demand during times of prolonged, recurrent changes in basal rate needs, such as during menses, when basal insulin needs may change for periods of time ranging from a few days to a few weeks.
Other conditions or situations can affect bolus insulin needs. Gastroparesis, a potential complication of diabetes resulting in slowed digestion, can alter postmeal insulin needs. Since digestion is slowed, insulin is needed for a longer duration following intake. Similarly, eating high-fat5-9 or high-protein meals5,9,10 can increase the amount and duration of bolus insulin needs postmeal. As a result, the meal bolus, as usually calculated, doesn’t always adequately cover insulin needs following high-fat and/or high-protein meals. The square-wave (SW) and dual-wave (DW) advanced features of insulin pump therapy are designed to deliver bolus doses over programmed periods of time and can accommodate these temporary alterations in bolus insulin need.
Features of Advanced Insulin Pump Therapy
Temporary Basal Rate
The temporary basal rate feature on insulin pumps incrementally changes the amount of basal insulin delivered for a specified period of time (from 30 minutes to 24 hours) to accommodate transient changes in basal insulin needs. The individual programs the pump to deliver a temporary basal rate that’s an increase or a decrease in his or her usual basal rate profile over a specified period of time. Once this time period ends, the usual basal rate profile automatically resumes.
An example of a temporary basal rate is shown in Figure 2.
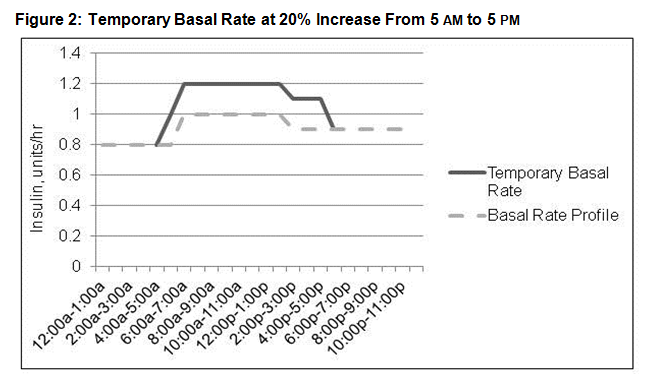
Here, the usual basal rate profile delivers 0.8 u/hr from midnight to 6 AM, 1 u/hr from 6 AM to 2 PM, and 0.9 u/hr from 2 PM to midnight. The temporary basal rate is set to deliver a 20% increase from 5 AM to 5 PM, equaling 1 u/hr from 5 to 6 AM, 1.2 u/hr from 6 AM to 2 PM, and 1.1 u/hr from 2 to 5 PM.
Alternate Basal Rate Profile
An alternate basal rate profile can be developed and stored in the pump for use on demand during times of prolonged, recurrent changes in basal rate needs. The individual turns the alternate basal profile on or off, switching back and forth from the usual basal profile to the alternate profile as needed. Most pumps allow at least four different basal rate profiles to be stored for use.
SW and DW Boluses
A normal meal bolus is delivered immediately, while an SW bolus is delivered over a specified period of time, and a DW bolus is a combination of a normal and an SW bolus in which part of the bolus is delivered immediately (as a normal bolus) and the remainder is extended over time (as an SW bolus).
In SW and DW boluses, the extended portion of the bolus may be delivered over 30 minutes to eight hours. In DW boluses, the amount of the bolus delivered as a normal bolus and the amount extended varies and is based on individual needs.
Figure 3 shows normal, SW, and DW boluses.
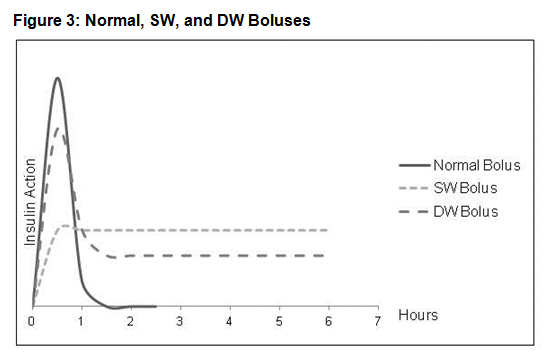
In this example, the SW bolus is extended over six hours, and the extended portion of the DW bolus stretches over six hours.
Using Advanced Features
Studies investigating insulin pump therapy have shown that individuals who give more boluses per day11-13 and those who program temporary basal rates more often12,13 have lower average BG levels. Research investigating the use of SW and DW features has shown that a prolonged delivery of insulin following meals can improve BG control.7-9,14
The Chase study included nine individuals wearing pumps who were aged 14 to 28 with type 1 diabetes and who consumed the same meal on four occasions one week apart: weighed quantities of pizza and tiramisu and measured amounts of nondiet cola. The meal consisted of approximately 11% protein, 53% carbohydrate, and 36% fat with 829 kcal. Subjects were asked to consume the meal within 20 minutes and remained in the testing center without exercising or smoking during the periods of study.7
The amount of bolus given at each meal was based on each subject’s individual ICR and was kept constant, but a different type of bolus was given at each meal: a normal bolus, two separate normal boluses (each providing 50% of the total bolus dose, with the second bolus given 90 minutes postmeal), an SW bolus extended over two hours, and a DW bolus with 70% given immediately and 30% extended over two hours. Table 2 outlines the study design.7
Mean BG levels were significantly lower at four hours postmeal for the DW and SW treatments compared with the normal and double-bolus treatments. The DW bolus delivery had the lowest change in BG levels from baseline than did all other delivery methods. The incidence of hypoglycemia was similar for the four methods of bolus administration.7
In a study by Jones et al, 24 adults with type 1 diabetes wearing pumps ate the same pizza meal on three consecutive evenings: plain cheese pizza from Pizza Hut with water. The size of the meal was based on each subject’s individual estimate of the amount they would typically consume (usually two to three slices) and was constant for each individual on the three successive nights. The study stated that an average slice of Pizza Hut pizza is 3.96 oz and provides 30 g CHO, 14 g fat, and 15 g protein (equaling 39% CHO, 41% fat, and 20% protein). Meal bolus amounts were based on individual ICRs and kept constant. However, the type of bolus varied: a normal bolus, a DW bolus with 50% given immediately and 50% extended over four hours, and a DW bolus with 50% given immediately and 50% extended over eight hours. Table 3 outlines the study design.8
The eight-hour DW bolus resulted in a greater percentage of time spent in the normal BG range (70 to 140 mg/dL) compared with the normal and four-hour DW boluses. In addition, mean BG was lowest for the eight-hour DW bolus compared with the other bolus methods in the eight- to 12-hour postmeal period. Mean BG levels below range didn’t markedly differ between regimens.8
It’s understood that meal-related insulin dosing based on CHO counting alone doesn’t always explain postmeal BG excursions, particularly with calorie-rich meals with a high-fat and/or high-protein content.5-10,14,15 The Pankowska method employs CHO plus fat/protein (CFP) counting to calculate meal bolus.16 An overview of the Pankowska method is shown in the sidebar at the end of this article. This method takes into account the effect of fat and protein digestion on postmeal BG elevations, though few studies have investigated it.
The Pankowska study involved 24 individuals with type 1 diabetes wearing pumps (mean age of 15) who were randomly assigned to two groups. Both groups ate a pizza meal that contained 46.8 g of CHO, 33.1 g of fat, and 25.4 g of protein (equaling 32% CHO, 51% fat, and 17% protein). One group employed CFP counting and received a DW bolus with the Pankowska method, and the other group employed CHO counting and received a normal bolus. Table 4 outlines the study design.9

The CFP+DW method resulted in lower average glucose levels at two to six hours postmeal, and differences in hypoglycemic events weren’t significant between the groups.9
Another study involved 42 individuals with type 1 diabetes wearing pumps (mean age of 12) who ate a pizza test meal on four different days containing 50% CHO, 34% fat, and 16% protein, corresponding to 33% of their age-adjusted daily energy requirement. They counted CHO at two meals and counted CFP at the other two meals. One CHO-counting meal employed a normal bolus, and the other used a DW bolus given as 70% immediately and 30% extended over two hours. One CFP-counting meal employed a normal bolus, and the other used a DW bolus with the Pankowska method. Table 5 outlines the study design.14
Postmeal BG was significantly lower with CFP counting than with CHO counting, independent of the kind of bolus used. Combining normal bolus with CHO counting led to the longest time of postmeal hyperglycemia (greater than 180 mg/dL).
Postmeal hypoglycemia (below 70 mg/dL) occurred significantly more frequently with CFP counting than with CHO counting. Using the normal bolus, 12 participants experienced hypoglycemia with CFP counting compared with five participants with CHO counting. When using a DW bolus, 18 participants experienced hypoglycemia with CFP counting compared with three participants with CHO counting.14
Kordonouri and colleagues suggest that the significant increase in hypoglycemia incidents when using CFP counting probably was due to the presence of a high proportion of basal insulin, and they assume that the study would have benefited from a run-in period to assess the accuracy of the existing individualized ICR and basal rates. It’s recommended that patients check the appropriateness of their basal rate settings (as discussed in the next section) before they start using CFP counting.14
The possibility that the Pankowska method overcalculates the insulin bolus can’t be excluded based on that study, but other studies9,15 didn’t show a significant increase in hypoglycemia incidents when using the method. It has been suggested to use the Pankowska method after a mixed meal once per day (eg, during the main meal or at dinner) to control late and prolonged hyperglycemia overnight.14
Counseling Patients
Some individuals may not be interested in or capable of employing advanced features of insulin pump therapy. Dietitians should use their best judgment before employing new features or making changes to pump settings. Characteristics that may help individuals successfully use advanced features include the following:
• can adequately navigate pump menus with button pushing;
• can verbalize the difference between basal and bolus insulin dosing;
• can’t reach BG goals with accurate use of pump therapy (ie, checking BG regularly, counting CHOs, accurately bolusing for meals);
• can keep scheduled pump therapy appointments; and
• can keep CHO, food, and BG logs when asked.
When using features of advanced pump therapy, basic rules of pump therapy counseling and management apply.
Avoid Hypoglycemia
When making adjustments to insulin pump settings, dietitians always should err on the side of caution to help individuals avoid experiencing hypoglycemia (BG below 70 mg/dL). It’s better to make smaller adjustments to pump settings, possibly resulting in BG levels higher than goal range, than to employ a drastic change and have individuals experience hypoglycemia.
Individuals should follow up by phone (if not in person) within 24 to 72 hours after changing a pump setting to be assessed for out-of-goal-range BG and to make adjustments as necessary.
Rule Out Noninsulin Variables
When considering an advanced pump therapy feature, dietitians first should rule out and then identify and correct noninsulin variables as the cause of out-of-goal-range BG levels. Noninsulin variables include miscalculation of CHOs, delayed or missed boluses, incorrect bolus administration (incorrectly programming the pump), hormonal effects, and growth spurts.
Introduce and Test One Feature at a Time
To keep track of which advanced pump therapy feature has been changed, should a patient’s BG levels become drastically affected, and to maintain control over it, dietitians should introduce only one feature at a time and test for accuracy before making another change. BG levels should be observed for three to seven days to assess accuracy. If hypoglycemia occurs, the change immediately should be reversed.
Basal Rate Testing
An individual’s BG response to basal rates should be tested by evaluating BG every one to two hours during various time segments: prebreakfast to prelunch, prelunch to predinner, predinner to bedtime, bedtime to overnight, and overnight to wake-up, or during the duration of a temporary basal rate. Time segments should be tested individually, with individuals abstaining from food and beverages during the test.
• Goal: BG doesn’t rise or fall more than 30 mg/dL during the test.
• If BG rises or falls more than 30 mg/dL, adjust basal rate by 10% to 20% for two to three hours before observed rise or fall; retest.
• If BG falls below 70 mg/dL, stop the test, treat low BG, and decrease basal rate by 10% to 20% for two to three hours before the observed fall; retest.
Testing SW and DW Boluses
Before testing SW and DW boluses, dietitians should work with individuals to answer these questions:
• Are boluses being administered on time? Boluses should be given before eating.
• Are CHOs (and fat and protein, if applicable) being counted correctly? Miscalculation of CHOs, protein, or fat will cause the administration of an incorrect bolus dose. It’s best to assess boluses when eating a meal in which the CHO, fat, and protein content are known.
Dietitians should compare an individual’s premeal BG with the two-hour postbolus BG, which is the BG two hours after the extended bolus has been delivered. For example, if an SW bolus was extended over four hours, the two-hour postbolus BG would be evaluated at six hours postmeal.
• Goal: Two-hour postbolus BG is 30 to 60 mg/dL higher than premeal BG.
• If two-hour postbolus BG is 60 mg/dL or more above the premeal BG, extend a larger portion of the total bolus next time; retest.
• If two-hour postbolus BG is 30 mg/dL or less than the premeal BG, extend a smaller portion of the total bolus next time; retest.
• If BG falls below 70 mg/dL at any time, stop the bolus, treat low BG, and give a smaller total bolus next time; retest.
In Conclusion
While individualized pump settings cover individual insulin needs, transient and recurrent changes in basal and bolus insulin needs do occur, occasionally warranting the use of advanced features in some individuals. Day-to-day cycles, dawn phenomenon, acute illness, stress, activity, menses, seasonal changes, and altered work schedules can affect basal insulin needs, while higher-fat and/or higher-protein meals or complications such as gastroparesis can alter bolus insulin needs. The use of temporary basal rates, alternate basal profiles, and SW and DW bolusing are advanced features of insulin pump therapy that can address these issues.
Once again it is important to determine whether individuals are appropriate candidates for using advanced features. Characteristics lending themselves to the use of advanced features include the ability to navigate pump menus, understanding basal vs bolus insulin, and not being able to reach BG goals with accurate use of pump therapy. Dietitians should introduce and teach one advanced pump therapy feature at a time and assess its accuracy before moving on to another feature. Close follow-up and erring on the side of caution are recommended to avoid hypoglycemia.
When used appropriately, advanced insulin pump therapy features have been shown to improve BG levels.
— Micki Hall, MS, RD, LD, CDE, CPT, is a clinical assistant professor at the University of Oklahoma Health Sciences Center College of Pharmacy and a certified insulin pump trainer.
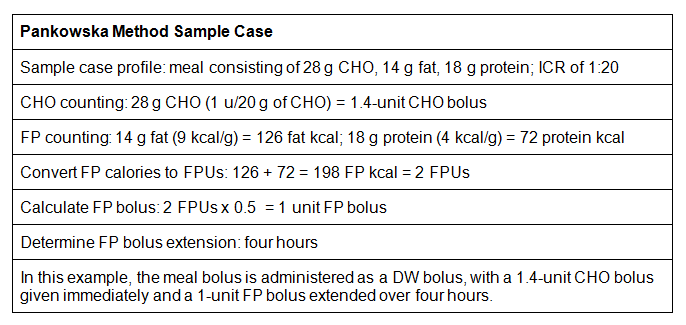
The Pankowska Method
References
1. Bolderman K. Putting Your Patients on the Pump. Alexandria, VA: American Diabetes Association; 2002.
2. Pickup J, Mattock M, Kerry S. Glycaemic control with continuous subcutaneous insulin infusion compared with intensive insulin injections in patients with type 1 diabetes: meta-analysis of randomized controlled trials. BMJ. 2002;324(7339):705.
3. Pickup J. Insulin pumps. Int J Clin Pract Suppl. 2011;(170):16-19.
4. Hovorka R. Closed-loop insulin delivery: from bench to clinical practice. Nat Rev Endocrinol. 2011;7(7):385-395.
5. Franz MJ. Protein: metabolism and effect on blood glucose levels. Diabetes Educ. 1997;23(6):643-646, 648, 650-651.
6. Boden G, Chen X. Effects of fat on glucose uptake and utilization in patients with non-insulin-dependent diabetes. J Clin Invest. 1995;96(3):1261-1268.
7. Chase HP, Saib SZ, MacKenzie T, Hansen MM, Garg SK. Post-prandial glucose excursions following four methods of bolus insulin administration in subjects with type 1 diabetes. Diabet Med. 2002;19(4):317-321.
8. Jones SM, Quarry JL, Caldwell-McMillan M, Mauger DT, Gabbay RA. Optimal insulin pump dosing and postprandial glycemia following a pizza meal using the continuous glucose monitoring system. Diabetes Technol Ther. 2005;7(2):233-240.
9. Pankowska E, Blazik M, Groele L. Does the fat-protein meal increase postprandial glucose level in type 1 diabetes patients on insulin pump: the conclusion of a randomized study. Diabetes Technol Ther. 2012;14(1):16-22.
10. Peters AL, Davidson MB. Protein and fat effects on glucose responses and insulin requirements in subjects with insulin-dependent diabetes mellitus. Am J Clin Nutr.1993;58(4):555-560.
11. Danne T, Battelino, T, Jarosz-Chobot P, et al. Establishing glycaemic control with continuous subcutaneous insulin infusion in children and adolescents with type 1 diabetes: experience of the PedPump Study in 17 countries. Diabetologia. 2008;51(9):1594-1601.
12. Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55(12):3155-3162.
13. Wilkinson J, McFann K, Chase HP. Factors affecting improved glycaemic control in youth using insulin pumps. Diabet Med. 2010;27(10):1174-1177.
14. Kordonouri O, Hartmann R, Remus K, Bläsig S, Sadeghian E, Danne T. Benefit of supplementary fat plus protein counting as compared with conventional carbohydrate counting for insulin bolus calculation in children with pump therapy. Pediatr Diabetes. 2012;13(7):540-544.
15. Carstensen S, Huber JW, Schönauer M, Thomas A. Minutes of the 45th General Assembly of the Europe-AN Association for the Study of Diabetes. Diabetologia. 2010;53(Suppl 1):403-404.
16. Pankowska E, Szypowska A, Lipka M, Szpotanska M, Blazik M, Groele L. Application of novel dual wave meal bolus and its impact on glycated hemoglobin A1c level in children with type 1 diabetes. Pediatr Diabetes. 2009;10(5):298-303.