For a printable PDF of this course and exam, click here.
Chronic kidney disease (CKD), which affects millions of people every year, can occur as a result of various diseases and health conditions. It also can occur when patients fail to take prescribed medications for chronic diseases or because of a poor financial situation that prevents patients from seeking treatment. Often, it can go undiagnosed because of poor medical follow-up by patients who have chronic medical conditions such as diabetes or hypertension.
It is the RD’s role to be aware of the underlying cause of a patient’s CKD and develop an individualized, evidence-based nutritional plan to improve the patient’s quality of life.
This continuing education course presents an overview of CKD and end-stage renal disease (ESRD), focusing on the causes of the disease and individualizing clients’ medical nutrition therapy by using the evidence-based guidelines established by the Academy of Nutrition and Dietetics (the Academy) and the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI).
National Statistics on Kidney Disease
In 2010, the National Kidney and Urologic Diseases Information Clearinghouse reported that more than 20 million Americans aged 20 and older had CKD. In 2009, the clearinghouse reported that more than 871,000 people were being treated for ESRD, more than 90,000 of whom died. The estimated cost for such treatment in 2009 was more than $42 billion. The survival rates for patients with ESRD (all treatment modalities) at one, five, and 10 years were 95.5%, 81.4%, and 10.5%, respectively.1
Every year the US Renal Data System collects data on patients with ESRD. According to its records, in 2009, there was a 3.3% increase in the treatment of ESRD by hemodialysis, peritoneal dialysis, transplants, and other approaches.2 From 2008 to 2009, the incidence of ESRD among Asians, African Americans, Native Americans, and Caucasians increased by 8.3%, 1.1%, 9.9%, and 3.5%, respectively.2
Also during that year, the percentage of ESRD patients with a primary diagnosis of diabetes increased by 2.5%; the percentage with glomerularnephritis increased by 1.2%; and the percentage with hypertension increased by 3.7%. However, the percentage of patients with a primary diagnosis of polycystic kidney disease decreased by 0.9%.
Thus, RDs need to pay particular attention to the follow-up and ongoing nutritional care of their Asian and Native American patients as well as those with poorly controlled diabetes, glomerularnephritis, and hypertension to improve five- and 10-year mortality rates.
Kidney Function and CKD
RDs can help slow the progression of CKD by managing a patient’s diet to reduce protein intake and by managing underlying health conditions such as diabetes, hypertension, and HIV. Because protein intake increases renal blood flow and glomerular filtration rates (GFRs), it’s important to decrease protein intake to help maintain lower blood flow and thereby reduce kidney damage.3 To help their patients, RDs need to know the kidney’s role, the five stages of CKD, and how the disease affects kidney function.
The kidneys are essential to life through three functions: excretory, endocrine, and metabolic.
Excretory function: The kidneys regulate total body water equilibrium by maintaining the inorganic-ion balance and by excreting metabolic wastes and foreign chemicals; they maintain this balance by producing urea and allowing the body to eliminate it.3-5
Besides regulating water homeostasis, the kidneys, during prolonged states of starvation or fasting, use lactate, pyruvate, amino acids, glycerol, free fatty acids, and beta-hydroxybutyrate to synthesize up to 45% of new energy in the form of glucose.
Endocrine function: The kidneys manage various endocrine responses that regulate blood pressure, bone metabolism, and the production of red blood cells. Additionally, when someone develops hypotension, the kidneys secrete the hormone renin, which helps convert angiotensinogen to angiotensin II, a potent vasoconstrictor that brings blood pressure back to normal. The kidneys also produce erythropoietin, which the bone marrow uses to produce the red blood cells needed to deliver oxygen to vital organs.3-5
Metabolic function: The kidneys produce active vitamin D3, which promotes calcium uptake in the small intestine and acts as an essential substrate for bone remodeling and maintenance.5
Five Stages of CKD
There is no cure for CKD. RDs should know how the disease progresses from one stage to the next and be able to identify what stage a patient is in to develop an individualized nutrition plan.6
Stages 1 and 2: Kidney disease is relatively unrecognized in stages 1 and 2 because there typically are no symptoms. Stages 1 and 2 generally are diagnosed when there is increased creatinine or urea in the blood, blood and/or protein in the urine, a family history of polycystic kidney disease, or evidence of kidney damage on radiologic exams.6
Stage 3: As patients progress to stage 3, they will experience uremia, anemia, high blood pressure, and slight metabolic bone disorders. These disturbances will lead to fatigue, fluid accumulation, decreased urine output, sleep disturbances, and kidney pain.6
Stage 4: As patients progress to stage 4, uremia, anemia, high blood pressure, and bone disorders become more prominent. The disturbances seen in stage 3 worsen and lead to additional complications of nausea, changes in taste, uremic breath, decreased appetite, neuropathy problems, and mental concentration issues.
At this stage, patients develop uremia because of the endocrine and metabolic changes that occur. Later, patients develop osteodystrophy, anemia, oxidative stress that leads to heart and vascular diseases, impaired immune function, and protein energy malnutrition as a result of inflammation from oxidative and carbonyl stressors.
Patients in stage 4 might complain of weakness, malaise, poor sleeping habits, fatigue, and loss of appetite caused by an increased amount of waste products in the blood. These waste products can lead to gastrointestinal disturbances that can result in poor food consumption, which in turn cause weight loss and the symptoms described above.3 Patients at this stage will be referred to a nephrologist for quarterly medical appointments to track disease progression. It is at this point that they start receiving information about dialysis or transplant.6
Stage 5: In stage 5, the patient has reached full kidney failure. Together with the metabolic and endocrine disorders seen in stage 4, the patient will have little to no urine output and can experience itching, muscle cramping, changes in skin color, and increased skin pigmentation. Patients might have weakness, malaise, poor sleeping habits, fatigue, and loss of appetite because of increased waste products in the blood, which can result in gastrointestinal problems, weight loss, and symptoms seen in other stages.3 Unless patients undergo a kidney transplant, they are given options for different types of dialysis treatment or hospice/palliative care.6
GFR’s Role
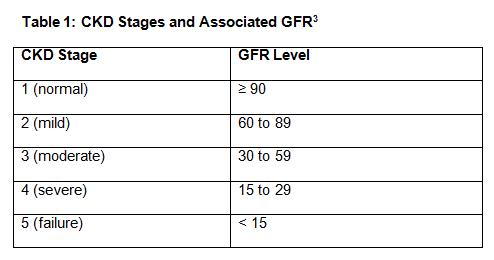
The presence of kidney disease is measured through the GFR, which gauges the patient’s level of kidney function. CKD is defined by a GFR below 60 mL/min/1.73 m2 with or without the evidence of kidney damage. This damage can be seen as albuminuria with levels greater than 30 mg of albumin on a urinalysis. Kidney failure is defined by a GFR below 15 mL/min/1.73m2.
Table 1, below, shows the different stages of CKD based on the various GFR values.
Causes of Kidney Disease
Kidney disease can be attributed to several underlying causes, some of the most common being nephrotic syndrome, glomerularnephritis, acute renal failure, diabetes, hypertension, and HIV. When aware of these conditions, RDs are better equipped to provide individual, evidence-based nutritional guidance for their patients.
Nephrotic syndrome: This is a loss of protein through the glomerular lumen, which can lead to proteinuria, hypoalbuminemia, edema, increased cholesterol, poor bleeding times, and alterations in bone metabolism. Most cases of nephrotic syndrome result from diabetes, lupus, amyloidosis, minimal change disease, membraneous nephropathy, focal glomerulosclerosis, and membranoproliferative glomerulonephritis.5
Glomerularnephritis (nephritic syndrome): This is an inflammatory response in the glomerulus capillary loop. It normally occurs only after streptococcal infections, and can cause hypertension and blood in the urine along with decreased renal function. The main side effect of this disease is hematuria.5
Acute renal failure: This develops when filtration rate and urea production suddenly drop, a process that can be reversed if caught in time. It usually occurs because of inadequate renal cell perfusion, a disease of the parenchyma cells in the kidney, or an obstruction of the urinary tract often seen with kidney stones.5
Diabetes: People with poor glycemic control from diabetes often experience increased thirst and will drink more fluids. As blood sugars continue to rise, the damage to the small blood vessels in the kidney increase with time.5
Hypertension: Poor blood pressure control places continued high pressure on the kidneys’ arteries and weakens them.5
HIV: Patients with HIV may be taking nephrotoxic drugs to help combat the infection.5 This can lead to lactic acidosis, crystal-induced obstruction, interstitial nephritis, and electrolyte abnormalities. The HIV infection can affect the cells in the kidney and also can attack the nephrons within the kidneys that help filter the by-products.6
How Subjective Global Assessments Determine a Patient’s Nutrition Status
Malnutrition is a common problem in most late-stage CKD patients because of the metabolic and endocrine disturbances that lead to poor appetite and weight loss. Thus, the Academy recommends that RDs perform subjective global assessments (SGAs) of their CKD patients at the initial visit and again quarterly to determine the patients’ nutrition status. SGA evaluations, which show whether any changes in nutritional status have occurred throughout the course of the disease, are critical for identifying patients who are nutritionally compromised in any stage of CKD or in danger of becoming malnourished.
SGAs merge both historical and physical data. The historical data can be gleaned from the past six months or even the past week and include weight and appetite changes, gastrointestinal alterations, ability to complete activities of daily living (functional status), and medical history (in particular, signs of fever, steroid use, and hypermetabolic diseases).
The physical aspects of SGA assess the loss of subcutaneous fat, muscle wasting, and edema on a four-point scale. The higher the SGA score, the more nutritionally compromised the patient is, with nutrition education being advised for scores of 2 or 3, and RD intervention for scores greater than 4. An example of the patient-generated SGA form can be found here.
Research during the past decade has supported the use of SGAs. Specifically, several articles that looked at malnutrition and mortality in patients with ESRD reported correlations with low SGA scores, which indicated malnutrition, and mortality rates increasing by as much as 500% based on the following factors: severity of malnutrition, an age greater than 55, dialysis treatment of fewer than two years, and the presence of diabetes.7-10
While this increase seems excessive, one study examined the interrater and intrarater reliability of RDs who received Web-based SGA training and reported moderate validity, with a 54% interrater reliability and a 68% intrarater reliability.11 Intrarater reliability is the reliability of a test producing the same result multiple times by the same researcher. Interrater reliability is the reliability of the test producing the same result among more than one researcher.
Lower scores of interrater reliability may be attributable to the differences in training among dietitians. Web-based training does not allow for actual hands-on experience, which can decrease the SGA’s reliability if RDs are not trained properly. Many dietetic programs now are having students and interns take hands-on approaches to learning SGAs or nutrition-focused physical exams, which are improving the reliability of RD assessments.
Some institutions have coach/mentors who accept visiting RDs for brief training periods to learn this technique. It is the RD’s responsibility to find local facilities in which to hold a physical training session. Also, a speaker could attend a local dietetic association or staff meeting to educate members on the use of patient-generated SGAs or have nutrition-focused physical exams with interactive participation to increase learning.
Another study looked at the specificity (malnutrition) and sensitivity (malnourished) of SGAs and determined that while they were only 32% specific in identifying patients with malnutrition, they were 100% sensitive in identifying those patients.12 For example, out of 100 patients, SGAs identified 16 patients as having malnutrition even though there might have been 50 patients. However, of the 16 patients identified as having malnutrition, SGAs were 100% accurate in defining these patients as malnourished.
This same study determined that by screening all dialysis patients for malnutrition on the basis of low serum albumin scores, a BMI of less than 18.5 and a greater-than-10% weight loss over six months followed by an SGA, all patients with malnutrition would be identified.12
By using the SGA, many studies reported that CKD patients, whether or not on dialysis, met only 50% to 70% of their energy needs and only about 50% of their protein needs.13-15 These numbers and SGA scores improved if RDs followed their patients monthly. The main causes of malnutrition in these patients were poor nutrition statuses (both energy and protein intakes), inflammation, age, and comorbidities leading to loss of lean body mass.14,16
Current Research on Why Nutrition Is Critical for CKD Patients
There have been several studies analyzing the importance of good nutrition for CKD patients. As indicated in the preceding section, depending on what stage of CKD the patient is in, increasing energy intake and varying amounts of protein intake are important ways of decreasing malnutrition because decreased protein intake can lead to hypoalbuminemia, malnutrition, loss of lean body mass and weight, and poor appetite secondary to uremia.17 In addition, higher levels of serum albumin markers, which indicate excellent protein intake, were correlated with decreased complications from CKD, improved energy levels, and improved mental well-being.18
One study showed that CKD patients’ appetites improved by 40.7% after the patients received nutritional supplements and RD intervention for three months.19 One of the protein indicators (normalized protein catabolic rate) improved above 1 g/kg/day in patients receiving the nutritional supplement, while the rate in the control group decreased, although this difference was not significant.19 This is important since a normal protein catabolic rate of 1 to 1.2 g/kg/day indicates optimal nutritional intake in dialysis patients, while rates below 0.8 g/kg/day indicate possible malnutrition.20
Another study looked at the impact of nutrition counseling on protein and energy intake. Both the control and intervention groups were given initial RD education; however, the intervention group was allowed additional nutrition education as requested (not required by the trial). The results found only slight improvements in the intervention group, though not significant, in protein or energy intake with RD follow-up.20
The study’s authors recommended that “dietetic resources may be used to greater effect if concentrated on ensuring a nutrient-rich diet, optimizing vitamin and iron intake, and promoting good potassium and phosphorus control” instead of focusing on energy and protein intake alone. However, it should be noted that during this study, few participants in the intervention group actually opted for the additional education. This lack of controlled intervention, coupled with the control group receiving identical instructions in the beginning and at month 4, could be the reason no significance was found.20
It’s important for RDs to consider optimizing nutrition by using individualized specific nutrition therapy, especially when evaluating potassium and phosphorus effects on the body. One study determined that dialysis patients could safely be given protein supplements three times per week without affecting phosphorus levels or binder needs.21 This supplement led to increased protein and energy intake as well as improved quality of life and SGA scores.
Another study looked at the effect of calcium dialysate baths on inflammation and mortality in hemodialysis patients.22 The study found that patients who received high-calcium dialysate baths during hemodialysis treatment had more inflammation, which led to a 2.765 times higher risk of death than did those who received standard and low-calcium baths.22
Finally, a further study on potassium and mortality found that mortality increased significantly with an increased intake of potassium.23 Dialysis patients who consumed more than 2 g of potassium daily had a 2.5 times higher risk of mortality compared with patients who consumed less than 1 g of potassium daily.23
Thus, because of the small number of patients meeting their nutritional needs,13-15 RDs need to be consulted when patients are found to be at increased risk of malnutrition. The importance of RD involvement is further shown in Figure 1, below, which reveals the average albumin levels in each state.2
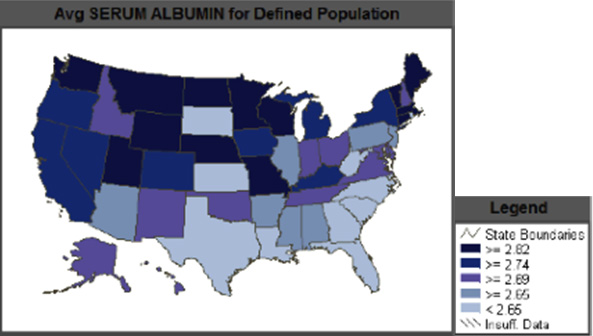
— Source: US Renal Data System, USRDS 2009 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, National Institutes of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland, 2009. The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the US government.
States with patients having albumin levels less than 3.5 g/dL should have nutritional education or direct intervention by an RD to improve these levels and, hopefully, decrease the risk of malnutrition in this population. While these numbers are from 2008 (the most current data available from the US Renal Data System), it’s easy to see which states fell below 2.8 g/dL, on average, for all CKD patients.
Evidence-Based Clinical Recommendations
The Academy and the KDOQI recommend that during each visit, RDs conduct an SGA and physical measurement of the patient, weigh the patient, check the patient’s recent lab results for protein and calcium, and monitor the patient’s fat, sodium, and fluid intake. RDs can do this by using the following six evidence-based guidelines:
1. Conduct a Nutrition Assessment and Initiate Nutrition Therapy
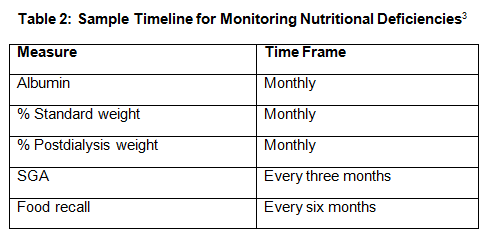
Both the Academy and the KDOQI recommend that when RDs assess CKD patients for malnutrition, they conduct an SGA or nutrition-focused physical exam (as previously mentioned) to determine patients’ nutrition status. Table 2, below, provides a suggested timeline for monitoring patients for nutritional deficiencies.
To prevent the progression of CKD and the onset of kidney failure, the Academy recommends that RDs initiate medical nutrition therapy, on referral from a licensed practitioner, for all patients with a CKD diagnosis because the best outcomes are achieved when RDs begin nutritional therapy as early as possible in the disease course.17 They should then follow up with the patient every one to three months and for one year if any nutritional deficiency presents itself, if the patient is experiencing malnutrition, and to follow the general course of the disease.17
2. Assess the Patient’s Energy Status and Caloric Intake
According to the Academy, energy needs should be based on a patient’s current weight, goals for his or her weight (ie, weight loss, gain, and maintenance), age, gender, level of physical activity, and any metabolic stressors (eg, pressure wounds, HIV infection, decompensated heart failure exacerbation).17
When assessing resting energy expenditure for any CKD patient, RDs should use an actual dry weight—that is, a weight that’s free of edema, ascites, and/or polycystic organs. It is usually the weight taken at the end of dialysis treatments.17 When patients are not on dialysis, the dry weight that should be used is the most current patient weight free of edema, ascites, and/or polycystic organs. Most often this will be the first weight in the morning taken after the patient has used the restroom. Any changes in body weight of 2 lbs in one day or 5 lbs in one week can indicate excessive body fluids.
While adjusted body weights are used for patients who are obese, they haven’t been validated for patients with CKD. As a result, RDs should monitor a patient’s lab results and weight on each exam to adjust energy intake if malnutrition begins to appear.
For stable CKD patients with a normal BMI, both the Academy and the KDOQI recommend the following guidelines17:
- 35 kcals/kg of actual weight for those younger than the age of 60;
- 30 to 35 kcals/kg of actual weight for those older than 60;
- 23 kcals/kg of actual weight to promote weight loss in patients who are overweight without fear of initiating malnutrition; and
- 50 kcals/kg of actual weight to promote weight gain in patients who are underweight or prevent weight loss during times of stress.
The KDOQI guidelines do not recommend increasing calories until the GFR is less than 25 or if the patient is in stage 4 CKD.
3. Determine the Patient’s Protein Intake
Protein intake can be affected by the type of protein—whether plant or animal—and the amount consumed. High biological value protein that’s found mostly in animal products (such as chicken, beef, pork, and fish), eggs, milk, quinoa, and soybeans can use the majority of nitrogen in a patient’s body, thereby decreasing urea production.
Low biological value proteins found in grains, nuts, dried beans, and peas produce more urea than do high biological value proteins because of incomplete amino acid profiles and should be limited. The goal for clinicians is to prescribe as many high biological value proteins as possible to maintain serum albumin levels around 4 g/dL. Also, as when conducting energy calculations, RDs need to consider a patient’s fluid status before making the calculations.17
If the patient has physician approval, exercise might minimize the catabolic effects of restricting protein and can improve the patient’s quality of life. Be aware that as protein intake is adjusted up or down, serum phosphorus levels might be affected since the majority of foods high in protein also contain a significant amount of phosphorus.
Although some international agencies recommend using keto-acid supplements in very low protein diets, the Academy and the KDOQI have not approved the use of such supplements for CKD patients.17 As a result, RDs should follow the Academy and the KDOQI recommendations for protein intake without using keto-acid supplements and low-protein diets.
While there are some differences between guidelines, the protein intake recommendations of both organizations can be found in Table 3, below.3,5,17
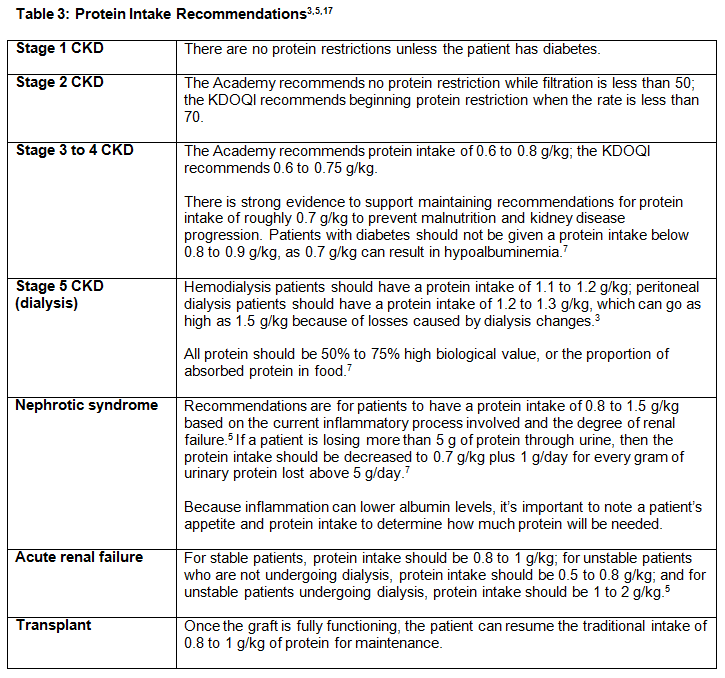
4. Prevent Renal Osteodystrophy and Osteomalacia
To prevent renal osteodystrophy, also known as metabolic bone disease, it’s important to maintain the levels of serum phosphorus, calcium, vitamin D, and parathyroid hormone (PTH).
RDs should help patients maintain phosphorus levels between 2.7 and 4.6 mg/dL for CKD stages 3 and 4 and between 3.5 and 5.5 mg/dL for stage 5.3
Phosphorus is found in almost all foods at some level, but the most common sources include protein foods, milk products, nuts, legumes, cereals, grains, dark cola, chocolate, cocoa, peanut butter, and beer. Patients who consume cereal will have reduced phosphorus absorption if they consume corn- and oat-based cereals because of their decreased phytase content.
Phosphorus therapy is used to treat secondary hyperparathyroidism, which causes renal osteodystrophy and potential soft-tissue calcification.17 Dietary restriction always is the first choice for therapy, and phosphate binders often are used to help lower levels when dietary restriction is not enough.
Typically, the use of binders would begin in stages 3 or 4 if serum phosphorus levels or intact PTH is elevated. RDs can adjust these binders with meals and snacks to reach the desired levels. Patients need to be aware that these binders only bind up to 300 to 400 mg/day with maximum dosages. If both dietary restriction and binders fail in the dialysis patient, the last choice of therapy is to increase the dialysis time or flow rate to improve serum levels.
Phosphorus guidelines from the Academy can be found in Table 4, below.17
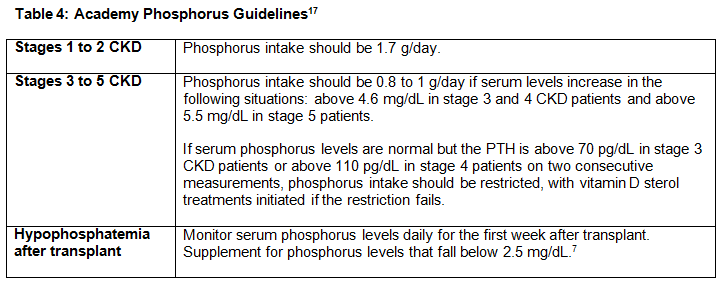
RDs also should consider calcium intake in metabolic bone disease. CKD patients should maintain calcium at normal levels between 8.4 to 9.5 mg/dL or the normal range for the laboratory used in all stages of CKD.3
The calcium/phosphate product (the product of the serum calcium level multiplied by the serum phosphate level) is a good way to determine whether a CKD patient has metabolic bone disease. The goal is to stay below a product of 55. If the product is greater than 70, this indicates the presence of osteodystrophy and an increased risk of soft-tissue calcification, especially if the serum PTH is elevated. If calcium is administered when the serum phosphorus level exceeds 5.5 mg/dL, the risk of soft-tissue calcification increases.
Calcium absorption can be altered by age, vitamin D status, gastrointestinal transit times (eg, gastroparesis, celiac disease, irritable bowel disease), decreased phosphorus intake, and decreased stomach acids as seen with peptic ulcers. Good sources of calcium include dark-green leafy vegetables, sardines, clams, oysters, canned salmon, soybeans, rhubarb, spinach, chard, fortified orange juice, milk products, and antacids.24 Vitamin D guidelines from the Academy can be found in Table 5, below.17

Although osteomalacia (softening of the bones because of a lack of vitamin D or the body’s inability to break down and use this vitamin) is uncommon in CKD patients, it can occur. RDs need to be aware of current practice recommendations, although the majority of the recommendations from the Academy’s Evidence Analysis Library are opinion based and should be used with caution.
Because of the lack of vitamin D resulting in elevated PTH levels, RDs should increase serum phosphate levels, within reason, by liberalizing the diet and adding vitamin D2 or D3 to improve bone markers.3,17 Osteomalacia guidelines from the Academy are as follows17:
- For stages 1 to 4, treat with phosphorus and vitamin D2 or D3 supplements.
- For stages 1 to 4, increase phosphorus supplements until serum levels normalize.
- If this treatment fails in stage 5 CKD patients, consider treating with vitamin D sterol.
5. Suggest Recommendations for Fat, Sodium, Potassium, and Fluid Intake
Fat intake: There are no studies looking at fat modification’s effect on CKD progression. However, there is limited evidence to recommend a low-fat (30%), low-cholesterol (fewer than 300 mg) diet in transplant patients with elevated fasting lipids. There also is preliminary evidence that fish oil and omega-3 fatty acid supplements decrease oxidative stress and improve fasting lipids in CKD patients.17
While current recommendations suggest that all CKD patients follow a low-fat, low-cholesterol diet to improve elevated fasting lipid levels, diet alone may not always work. In that case, as an adjunct therapy, omega-3 supplements can be added.
Sodium intake: The following guidelines are recommended for sodium intake in CKD patients3,17:
- Stages 1 to 4 CKD: 1 g to 3 g/daily
- Stages 1 to 4 CKD with hypertension: Fewer than 2.4 g/daily
- Stage 5 CKD on dialysis: 750 mg to 1,000 mg/daily
- Transplant: Fewer than 2.4 g/daily
Adjust and monitor sodium levels based on blood pressure, medications the patient is taking, kidney function, hydration status, acidosis, glycemic control, catabolism, and gastrointestinal complications (nausea, vomiting, and diarrhea). Sources of high levels of sodium include salt, processed foods, and sodium bicarbonate therapy. A discussion of sodium with your patient is not complete without referencing fluid intake (see below).
Potassium intake: RDs should monitor potassium levels because a low level can cause muscle cramps and cardiac arrhythmias, the latter of which can lead to death.
Serum potassium levels can rise not only after the patients have consumed certain foods, such as bananas, but also with the use of antihypertensive medications (eg, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers) and from poor glycemic control, which leads to potassium being pulled out of cells from the high osmolarity of sugar in the bloodstream.3
Evaluate medications and glycemic control if a patient presents with hyperkalemia but has optimal potassium intake. Advise people with diabetes to treat hypoglycemia with cranberry, grape, or apple juices; glucose tablets; or 10 small jelly beans instead of using high-potassium alternatives such as orange juice.
RDs should educate patients to avoid salt substitutes, milk, potatoes, coffee, tomatoes, and orange and grapefruit juices to help regulate their potassium levels. If patients find it difficult to give up potatoes, RDs should recommend soaking the potatoes to decrease potassium content and slice them in a way that maximizes the surface exposure to large volumes of water. Advise patients to soak potatoes at room temperature for at least 30 minutes and then boil them in a large volume of water. This might decrease the potassium content down to 100 to 200 mg per 1/2-cup serving.
To maintain potassium levels between 3.5 and 5.5 mEq/L, patients should consume at least 4 g of potassium daily for stages 1 and 2 CKD or fewer than 2.4 g daily for stages 3 to 5 and transplant recipients.
If a patient develops skeletal muscle cramps associated with potassium alterations and dialysis treatments, RDs may want to recommend carnitine supplements since the kidneys are the main site for carnitine synthesis.5 Carnitine facilitates the transfer of long-chain fatty acids into cellular structures, including mitochondria,3 and these acids are the major fuel source for skeletal and myocardial muscles. When patients develop CKD, carnitine synthesis decreases, and muscles can cramp because of inadequate fuel. Carnitine supplementation may increase free and acyl carnitine levels, improve a patient’s capacity for physical activity, and decrease dialysis-related symptoms, including muscle cramps.
However, RDs should use carnitine only in patients with disabling muscle weakness, cardiomyopathy, or hypotension when current treatments fail to work. Oral supplementation is 0.8 g/day and can be less expensive than some medical treatments; however, the intestinal absorption of the carnitine is unpredictable. If supplementation is indicated in stage 4 or 5 CKD, RDs can infuse 10 to 20 mg/kg of carnitine at the end of dialysis treatments three times per week. If there is no improvement at the end of three to six months, RDs should discontinue the carnitine supplement.17
Fluid intake: Fluids are important to consider in nutrition assessment, as they can alter the outcome of medical nutrition therapy in CKD. Since the kidneys are responsible for water homeostasis, as stated above, patients with CKD either can become edematous from too much fluid intake or dehydrated from too little intake while on diuretics for blood pressure control.
Weight changes can affect how RDs assess calorie and protein intake. As previously mentioned, it’s important to know from the outset the actual dry weight of a CKD patient to accurately calculate nutrition requirements. The amount of fluid intake differs greatly depending on the patient’s stage of CKD. Consider the following:
- Stages 1 to 4 CKD: Unless specified by a physician, patients with stages 1 to 4 CKD do not have to limit their fluid intake if they do not have other comorbid conditions, such as heart failure or polycystic kidney disease. Fluid restrictions for CKD patients with these conditions can be from 1,500 to 3,000 mL/day based on a patient’s dry weight.3,5,17 Maintaining proper fluid intake in these stages is important because CKD patients are taking diuretics to control blood pressure and need to maintain as much kidney function as possible.
- Hemodialysis: Since hemodialysis patients undergo dialysis only three times per week, it’s important that they don’t consume excessive fluid, which could lead to pulmonary edema or congestive heart failure. Two sources recommend that hemodialysis patients consume only 1,000 mL of fluid plus an equal amount for the urine they produce.3,17 Another source suggests they consume only 750 to 1,500 mL daily.5
- Peritoneal dialysis: Because peritoneal dialysis patients typically dialyze every day,3 their fluid intake is not as restricted as it is for hemodialysis patients. Cyclic peritoneal dialysis patients should consume 1,000 mL of fluid plus the equivalent of urine output. Continuous ambulatory peritoneal dialysis patients should consume 2,000 mL of fluid plus the equivalent of urine output.5
- Nephrotic syndrome: There are no fluid restrictions for patients with nephrotic syndrome at this time.5
- Transplant: There are no fluid restrictions for transplant patients at this time.5
Most patients know about sodium restriction and fluid intake associated with the sodium and fluid guidelines above. However, they often are surprised to learn how much water actually is in the food they eat. RDs should educate patients about the water content in foods, especially during the summer months when people tend to eat more fruits and vegetables, which are high in water content.
Figure 2, below, provides a quick reference guide for nutrition therapy through the various stages of CKD.

6. Monitor Anemia in Patients With CKD
Patients with CKD have a high risk of anemia, not just from decreased erythropoietin production by the kidneys, but also because of inadequate food intake that contributes to micronutrient deficiencies.17
RDs should ask the patients’ physicians to order lab tests to determine the patients’ levels of vitamin B12, folic acid, iron, ferritin, and transferrin saturation to ensure that other micronutrient deficiencies are not missed. Consider instituting nutritional protocols for normal supplementation for vitamin B12, folic acid, and iron if patients are deficient in these nutrients.
If a patient’s iron level is low, the RD may want to consider supplementing the patient’s diet with vitamin C to help increase iron absorption, but be aware that vitamin C intake above normal recommendations can lead to kidney stones.17
Achieving patients’ compliance with recommendations for taking iron supplements may be problematic if the supplements cause constipation. RDs should talk with their patients about ways to minimize constipation, not with water because of their fluid restrictions, but with soluble fibers. One way to increase the iron content in foods is by cooking with iron pots or skillets. If an acidic food such as tomato sauce is added to an iron pot or skillet, iron can leach into the food (the newer the pot, the more iron that will leach). On average, heating tomato sauce in a new skillet can increase the iron content from 1 to 6 mg and applesauce from 0.5 to 7 mg. Note that this will not work with enamel-coated iron skillets, as the iron must come into direct contact with the food.
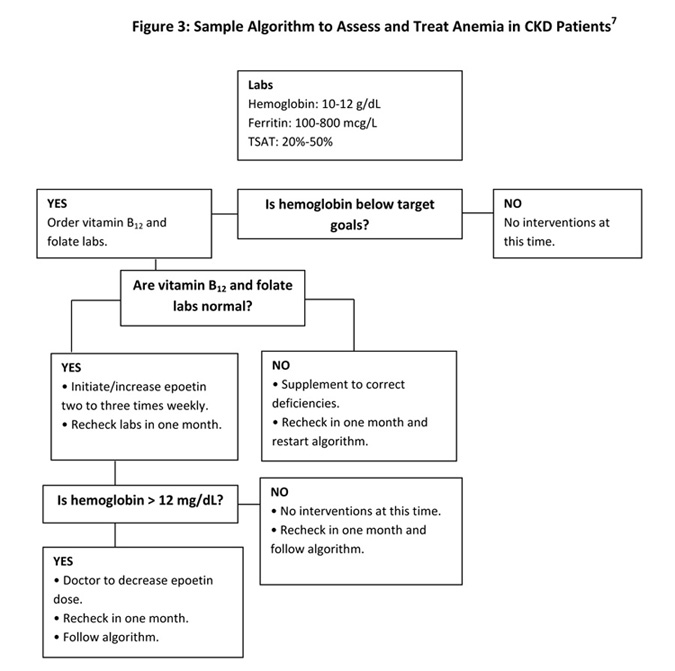
Target goals for anemia control are a hemoglobin level between 10 and 12 mg/dL, a ferritin level between 100 to 800 mcg/L, and transferrin saturation scores (TSAT) between 20% and 50%.17 RDs should never recommend that a patient begin epoetin alpha (Procrit, Epogen), the medication intended to improve anemia in CKD patients, until vitamin B12 and folate deficiencies have been ruled out. Once those deficiencies have been ruled out, RDs can recommend starting epoetin alpha two to three times per week.1 The patient’s physician should decrease epoetin alpha only if the hemoglobin level rises above 12 g/dL.
See Figure 3, below, for a guide to medical nutrition therapy guidelines for treating CKD patients who have anemia.
A Plan for the Future
As RDs continue to work toward more evidence-based practices, it’s important to stay current with all the literature and use the protocols and guidelines established by their governing bodies.
It’s also important for RDs to investigate the underlying causes of CKD to identify the proper nutrition therapy for a patient. For example, a patient with CKD resulting from diabetes will have a much different nutrition intervention than will one who has CKD resulting from hypertension or one with polycystic kidney disease.
Through evidence-based medical nutrition therapy and individualized nutrition plans, RDs can play a significant role in improving the quality of life for their CKD patients.
¾ Written by Kimberly Thompson, MS, RD, LDN, a clinical specialist dietitian for the Memphis VA Medical Center. She works with veterans in the spinal cord injury unit and with system redesign processes.
References
1. Kidney disease statistics for the United States. National Kidney and Urologic Diseases Information Clearinghouse website. http://kidney.niddk.nih.gov/KUDiseases/pubs/kustats/index.aspx. Last updated July 2, 2012. Accessed September 18, 2012.
2. United States Renal Data System website. http://www.usrds.org/render/ xrender.phtml. Accessed March 10, 2012.
3. Kopple JD. Nutrition, diet, and the kidney. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease. 10th ed. Philadelphia: Lippincott Williams & Wilkins; 2005:1475-1511.
4. Vander A, Sherman J, Luciano D. Human Physiology: The Mechanisms of Body Function. 8th ed. New York: McGraw-Hill; 2001:505-551.
5. Wilkens KG. Medical nutrition therapy for renal disorders. In: Mahan LK, Escott-Stump S, eds. Krause’s Food, Nutrition & Diet Therapy. 10th ed. Philadelphia: Saunders; 2000:368-376.
6. Stages of kidney disease: what is end-stage renal disease? DaVita website. http://www.davita.com/kidney-disease/overview/stages-of-kidney-disease. Accessed July 27, 2012.
7. De Mutsert R, Grootendorst DC, Boeschoten EW, et al. Subjective global assessment of nutrition status is strongly associated with mortality in chronic dialysis patients. Am J Clin Nutr. 2009;89(3):787-793.
8. Segall L, Mardare N, Ungureanu S, et al. Nutritional status evaluation and survival in haemodialysis patients in one centre from Romania. Nephrol Dial Transplant. 2009;24(8):2536-2540.
9. De Mutsert R, Grootendorst DC, Axelsson J, et al. Excess mortality due to interaction between protein-energy wasting, inflammation and cardiovascular disease in chronic dialysis patients. Nephrol Dial Transplant. 2008;23(9):2957-2964.
10. De Mutsert R, Grootendorst D, Indemans F, et al. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J Renal Nutr. 2009;19(2):127-135.
11. Steiber A, Leon JB, Secker D, et al. Multicenter study of the validity and reliability of subjective global assessment in the hemodialysis population. J Renal Nutr. 2007;17(5):336-342.
12. Gurreebun F, Hartley GH, Brown AL, Ward MC, Goodship THJ. Nutrition screening in patients on hemodialysis: is subjective global assessment an appropriate tool? J Renal Nutr. 2007;17(2):114-117.
13. Cupisti A, D’Allesandro C, Valeri A, et al. Food intake and nutritional status in stable hemodialysis patients. Ren Fail. 2010;32:47-54.
14. Cheema B, Abas H, Smith B, et al. Investigation of skeletal muscle quantity and quality in end-stage renal disease. Nephrology. 2010;15:454-463.
15. Campbell KL, Ash S. Davies PSW, Bauer JD. Randomized controlled trial of nutritional counseling on body composition and dietary intake in severe CKD. Am J Kidney Dis. 2008;51(5):748-758.
16. Jahromi SR, Hosseini S, Razeghi E, Meysamie AP, Sadrzadeh H. Malnutrition predicting factors in hemodialysis patients. Saudi J Kidney Dis Transpl. 2010;21(5):846-851.
17. Chronic kidney disease evidence-based nutrition practice guidelines. Academy of Nutrition and Dietetics Evidence Analysis Library website. http://www.adaevidencelibrary.com/topic.cfm?cat=3927. Accessed February 20, 2012.
18. Mazairac AHA, de Wit GA, Penne EL, et al. Protein-energy nutritional status and kidney disease-specific quality of life in hemodialysis patients. J Renal Nutr. 2011;21(5):376-386.
19. Malgorzewicz S, Rutkowski P, Jankowska M, Debska-Slizien A Rutkowski B, Lysiak-Szydlowska W. Effects of renal-specific oral supplementation in malnourished hemodialysis patients. J Renal Nutr. 2011;21(4):347-353.
20. Sutton D, Higgins B, Stevens JM. Continuous ambulatory peritoneal dialysis patients are unable to increase dietary intake to recommended levels. J Renal Nutr. 2007;17(5):329-335.
21. Fouque D, McKenzie J, de Mutsert R, et al. Use of a renal-specific oral supplement by haemodialysis patients with low protein intake does not increase the need for phosphate binders and may prevent a decline in nutritional status and quality of life. Nephrol Dial Transplant. 2008;23:2902-2910.
22. Hsu CW, Lin JL, Lin-Tan DT, et al. High-calcium dialysate: a factor associated with inflammation, malnutrition and mortality in non-diabetic maintenance haemodialysis patients. Nephrology. 2010;15(3):313-320.
23. Noori N, Kalantar-Zadeh K, Kovesdy C, et al. Dietary potassium intake and mortality in long-term hemodialysis patients. Am J Kidney Dis. 2010;56(2):338-347.
24. Anderson JJB. Minerals. In: Mahan LK, Escott-Stump S, eds. Krause’s Food, Nutrition & Diet Therapy. 10th ed. Philadelphia: Saunders; 2000:113-117.