For a printable PDF of this course and quiz, click here.
Dysphagia is commonly defined as the presence of an abnormality in the passage of solids or liquids from the oral cavity to the stomach in any of the normal swallowing phases: oral, pharyngeal, and/ or esophageal.1 Any disturbance in the swallowing process may be considered dysphagia.
While dysphagia is a common condition in older adults, development of dysphagia is not an age-related condition.1 The deterioration in muscular structure associated with difficulty swallowing is largely a result of the comorbidities that are common in older adults.2 Patients with dysphagia experience substantial social and psychosocial afflictions related to their symptoms of difficulty swallowing. Anxiety with meals and avoidance of consuming meals in a social setting are often seen in patients with dysphagia.2
The presence of dysphagia can contribute to malnutrition, dehydration, respiratory infection including pneumonia, and overt as well as silent aspiration.2
The importance of timely diagnosis of dysphagia is heightened by the morbidity and mortality associated with pneumonia, for which those with dysphagia are at increased risk. A study by Muder reported that in long term care, the percentage of patients who experienced pneumonia-related mortality over the course of one year was 19% after 14 days, 59% after one year, and 75% two years after they had contracted pneumonia.3 A study conducted in a VA long term care facility yielded similar results. In that study, mortality was 23% at 14 days and 75% at the one-year mark.4 A prospective study by Serra-Prat and colleagues reported that in community-dwelling older adults, malnutrition is more prevalent in dysphagia sufferers than in older adults without swallowing dysfunction, at 18.6% and 12.3%, respectively.5 Older adults with signs and symptoms of dysphagia are at increased risk of comorbidities such as aspiration pneumonia.6 RDs play a vital role in the care of older adults with dysphagia. As part of the interdisciplinary team, they not only assist dysphagia sufferers to optimize nutrition and hydration status, but also to obtain a balance between patient safety and quality of life. RDs’ unique set of skills position them to manage dysphagia. RDs’ scope of practice activities that help in the screening and management of dysphagia include their role in assessing and managing unexplained weight loss, inadequate oral intake, difficulty chewing, and meal observations such as inability to control food in the oral cavity.
This continuing education course reviews the mechanics of swallowing, swallowing changes that occur as part of the aging process and their impact on the development of dysphagia, and nutrition interventions to mitigate the symptoms.
Prevalence
While the actual incidence of dysphagia across different care settings has not been determined, it is estimated that 15% of older adults suffer from dysphagia.7 Other literature suggests that the prevalence of dysphagia varies in relation to associated disorders, the segment of the population reviewed, and the diagnostic test used.8 For instance, dysphagia is believed to occur in 29% to 64% of stroke patients.9-11 In patients with other neurologic disorders such as multiple sclerosis and Parkinson’s disease, the prevalence of dysphagia fluctuates from 24% to 34%12,13 in the former and 81% in the latter.14 A population-based study conducted in 1997 reported that the prevalence of dysphagia was significantly higher in patients with gastroesophageal reflux disease (GERD), approximately 30%, when compared with 4% of subjects without GERD.15 Approximately 40% of older adults residing in health care facilities have been identified as having dysphagia.16
Healthy Swallowing
Oropharyngeal/esophageal swallowing function changes that occur as part of the normal aging process do not interfere with the older adult’s ability to maintain good health.17 Swallowing, or deglutition, is the process by which food, fluids, and other objects travel from the oral cavity to the pharynx and into the esophagus as the epiglottis closes. Swallowing is a complex process that requires precise coordination with breathing as both swallowing and breathing share the same entryway, the pharynx.18
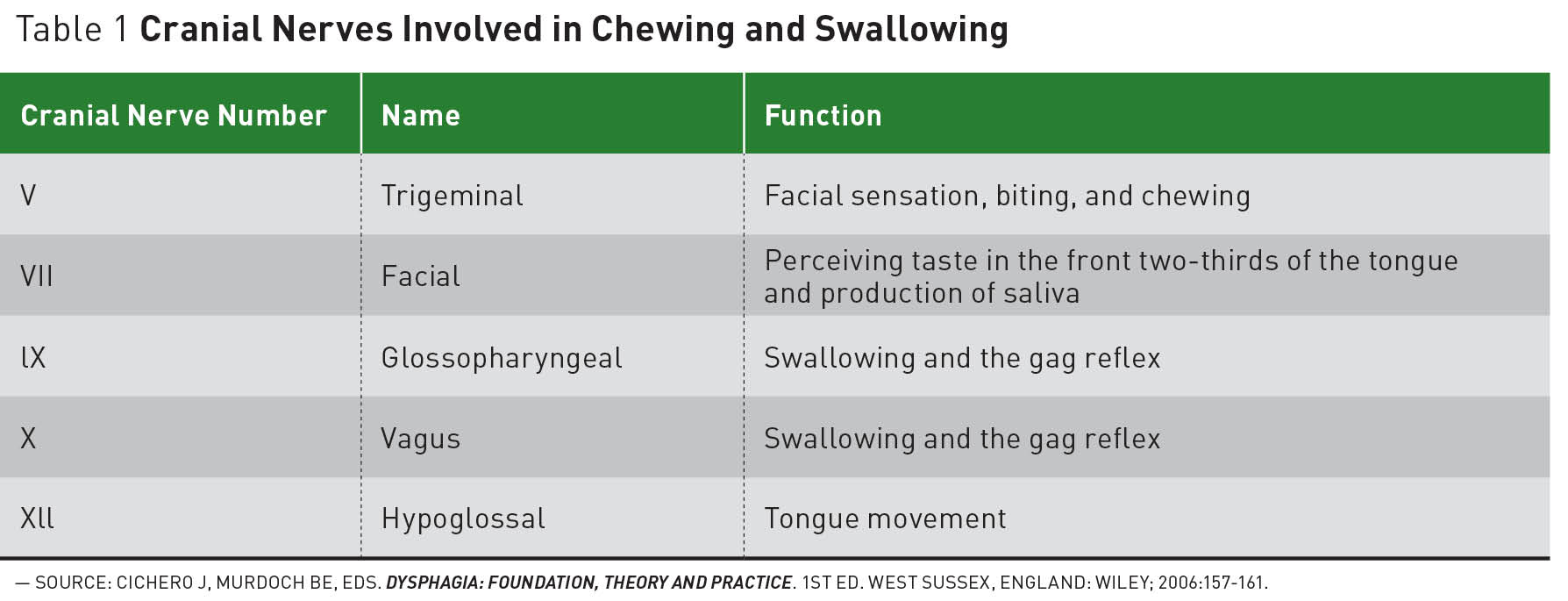
Prior to the actual swallowing motion, the mandible and the lips work to capture the food and fluid that enter the oral cavity. Once the food and fluid enter the oral cavity, the mandible rises and the lips adduct to contain them in the mouth. Food is mechanically ground by the teeth as the mastication muscles and the cranial nerve (CN) V, also known as trigeminal nerve, act on the temporomandibular joint to control the action. This results in a bolus, which is moved from one side of the oral cavity to the other by the tongue. The facial nerve, CN VII, helps to maintain the bolus against the occlusal surfaces of the teeth. The bolus is ready for swallowing when it is thoroughly moistened by saliva, involving CN VII and IX, and detected by the lingual nerve of the tongue. If food is too dry it will not form a bolus and cannot be swallowed.18 See Table 1 for a list of the cranial nerves involved in chewing and swallowing.

As previously stated, this synchronization allows the food to be mechanically altered to create a bolus and prepare the food to be swallowed. The process allows the swallow to move from the oral stage, in which the muscles in the mouth and tongue grind the food, to the pharynx, where the soft palate keeps food from entering the nose. The base of tongue, or the lowest part of the tongue, makes contact with the pharyngeal wall to generate pressure that drives the food through the pharynx and into the esophagus. The larynx must close to prevent food from entering the trachea, and the upper esophageal sphincter opens at the precise moment to allow food into the esophagus.18 These actions transpire within several seconds.
Well-timed coordination is essential in the swallowing process. The food must be thrust along with pressure produced by the tongue, the lowest part of the tongue, the base of tongue, in the pharynx, and the walls of the pharynx, which help contract consecutively, top to bottom, pushing the food to the esophagus where the esophageal muscles take over, contracting successively to push the food into the stomach.18
Swallowing and the Aging Process
Some changes in the swallowing function are considered normal aspects of the aging process. Conditions that impact normal swallowing are common in older adults;19 however, research indicates that impaired swallowing such as dysphagia occurs as a result of disease as opposed to occurring as a consequence of the normal aging process.20
Normal swallowing slows as individuals age, causing small amounts of food to linger in the throat a little bit longer than in life’s earlier stages. Normally, despite this slowing, the efficiency and/or safety of swallowing does not change significantly in healthy aging individuals.21,22
Swallowing difficulty can result from the use of medications, such as narcotics and anticholinergics, that affect the nervous system, mechanical distresses such as nasogastric and tracheostomy insertion, and chronic medical conditions (such as Parkinson’s disease).20 Changes that occur with aging such as tooth loss as well as shifting teeth that require foods to be mechanically altered affect the swallowing process. Other changes that contribute to increased effort required to swallow or interfere with swallowing safety include an impaired ability of the vocal cords to protect the airway. If the airway is not fully protected during swallowing, food particles can enter the lungs, a process called aspiration. Reduced strength in the tongue and pharynx interferes with the ability to form and move a bolus from the mouth to the esophagus. The upper part of the esophagus is a sphincter that relaxes to open and allow the passage of food and fluids. As individuals age, the size of their esophageal sphincters can decrease, causing food, liquids, and medications to become trapped or become difficult to swallow. During normal swallowing, airway protection occurs via the closing of the epiglottis. The longer swallowing time observed in older adults increases the length of time the airway is exposed due to slower epiglottis closure, thus increasing the risk for unsafe swallow.23
Dysphagia in the Elderly
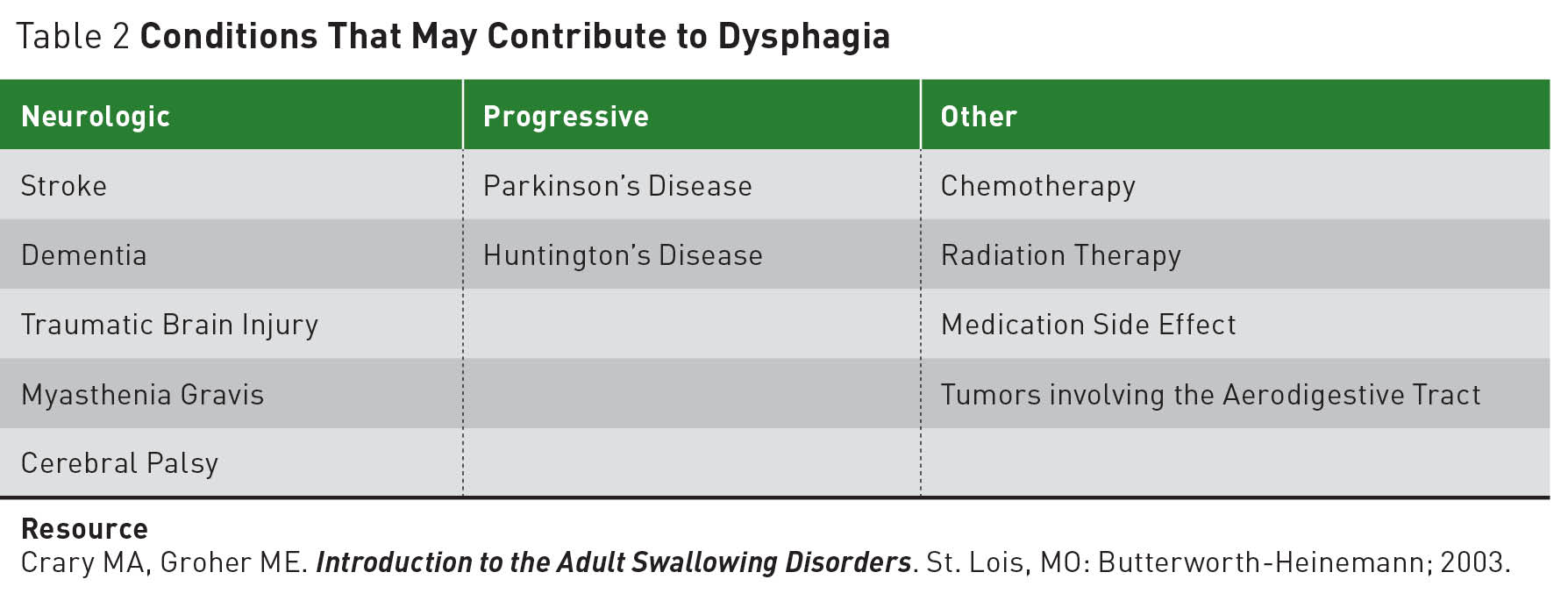
Dysphagia is an increasing health concern in the elderly population. Age-related changes in swallowing physiology coupled with age-linked chronic illnesses are predisposing elements for dysphagia in older adults. Due to the intricacy of the swallowing process, many diseases can affect the swallowing function. Table 2 provides some examples of conditions that can contribute to compromised swallowing function.
Depending on the area of the gastrointestinal tract affected, dysphagia can be categorized as oropharyngeal or esophageal.

Oropharyngeal Dysphagia
Oropharyngeal dysphagia is defined as difficulty clearing food and objects from the oropharynx into the esophagus.19 This results from irregular function proximal to the esophagus. Oropharyngeal dysphagia is common in older adults with neurologic conditions such as stroke, Parkinson’s disease, or multiple sclerosis.17 Muscular disorders that affect skeletal muscles such as muscular dystrophy or myasthenia gravis also can contribute to the development of oropharyngeal dysphagia.17
Esophageal Dysphagia
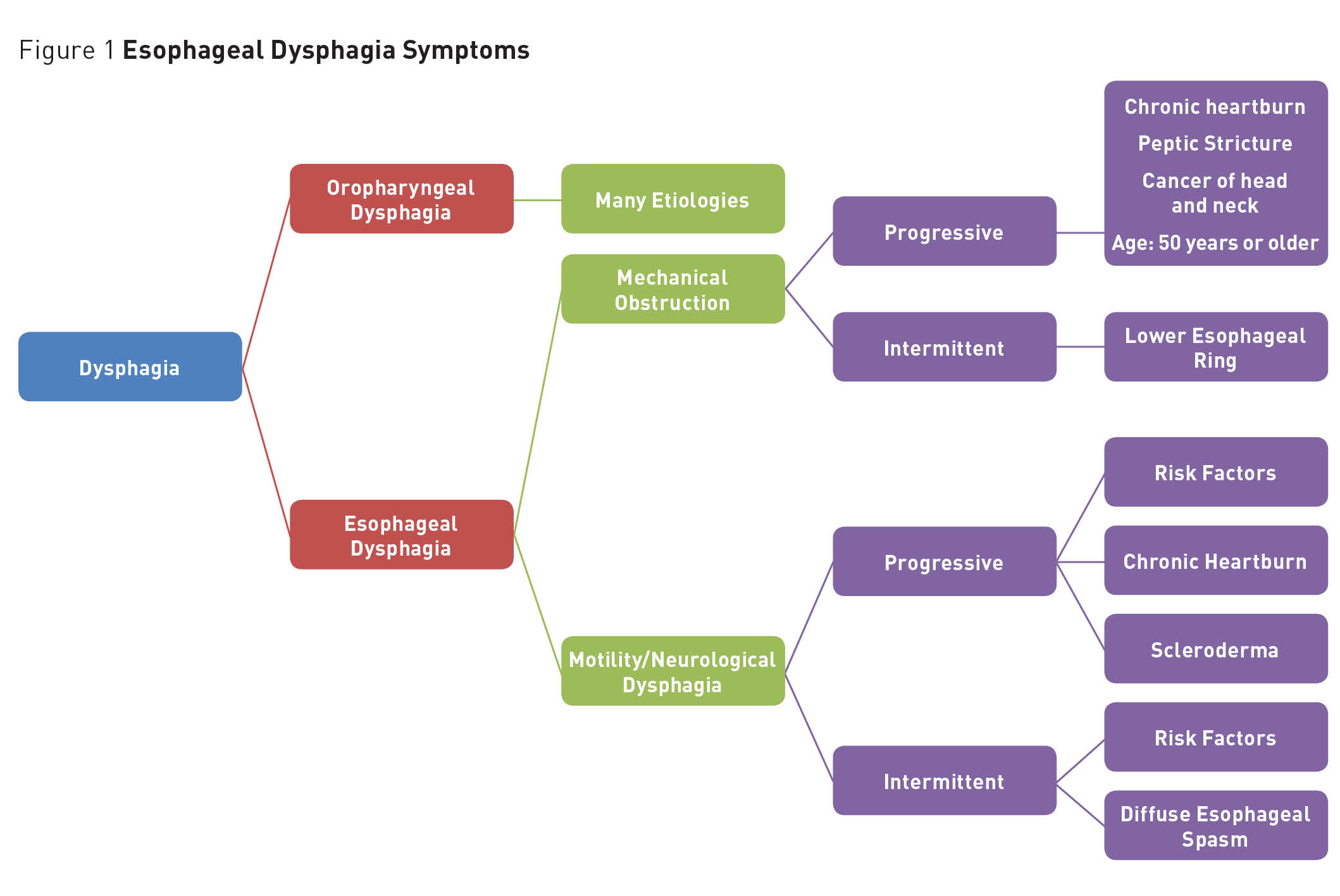
Esophageal dysphagia sufferers frequently describe the condition as the sensation of pressure in the chest area or difficulty forcing food into the esophagus. This can occur as a result of issues with motility or a mechanical obstruction and may be progressive or intermittent.17,19
Dysphagia resulting from motility problems is characterized by difficulty swallowing both solids and liquids. Dysphagia related to motility is a neuromuscular disorder that can be progressive or intermittent. Achalasia contributes to progressive motility dysphagia while diffuse esophageal spasms contribute to intermittent.17,24
Dysphagia resulting from a mechanical obstruction begins with difficulty swallowing solids and evolves to include difficulty consuming liquids.
Conditions common to those aged 50 or older, such as chronic heartburn, peptic stricture, and cancer result in a progressive mechanical obstruction that causes dysphagia. An intermittent mechanical obstruction is caused by dysfunction in the lower esophageal ring—a stricture abnormality of the esophagus.24 Figure 1 depicts signs and symptoms of esophageal dysphagia.

Dysphagia Signs and Symptoms
Older adults with dysphagia may demonstrate signs and symptoms such as coughing or choking before, during, or after meals and throughout the swallowing process. Difficulty controlling food and saliva in the mouth, difficulty initiating a swallow, nasal regurgitation, drooling, and spilling of food from one or both sides of the mouth are common. Patients often describe symptoms such as food and liquids “sticking” to their throat.23,25 Some older adults show no overt signs, but instead may have changes in their vocal quality, fever episodes after meals, and recurrent aspiration pneumonia.23,25
Diagnosing Dysphagia
The first step in evaluating swallowing dysfunction involves taking a detailed history to aid in pinpointing the cause of the swallowing difficulty. This is followed by a physical examination, which includes an assessment of the oral cavity, pharynx, and larynx. A trained health care provider skilled in the evaluation and treatment of dysphagia, usually a speech-language pathologist (SLP), examines the patient for range of motion of the tongue (lateral, vertical, protrusion) and muscles involved in swallowing, coordination, and speed of movement of the food through the oral cavity and the pharynx. Assessment also includes evaluation of the lips, palate, tongue, and larynx to ensure that the person can accept food in the oral cavity and that the larynx protects the airway during swallow.19,23
The assessment includes discussion of the duration and acuity of symptoms. It is important to identify the food and fluid items that produce difficulty and where discomfort is located (in the larynx or the esophagus). It is important to determine whether there is a difficulty swallowing solids, liquids, or both; any incidence of drooling or food spilling from the sides of the mouth; and coughing or choking during meals.19,23
The examination should include evaluation of nutritional status, including body weight changes and BMI. Neurologic assessment includes evaluating muscle strength and the cranial nerve function. According to a 1993 article by Bleach in Otolaryngology and Allied Sciences, noting an abnormal gag reflex has been used as an indicator of swallowing dysfunction. As a sole indicator of swallowing difficulty, this is an unreliable marker. To test for the presence of a gag reflex, the clinician asks the subject to open his or her mouth and examines the oral cavity with a penlight. The subject is then asked to say “Ah” as the clinician watches the pharynx and uvula. Then the clinician will observe the rise of the soft palate and the uvula as the “Ah” sound is made. The uvula should rise straight up in the midline of the oral cavity.19 The neck is assessed for presence of an enlarged thyroid or other mass.19,23 Head and neck cancers as well as an enlarged thyroid can be risk factors for dysphagia development.26
Diagnostic Testing
Based on an individual’s medical history and a physical examination, tests can be ordered by the physician to observe the exact appearance and coordination of movement of the structures of the oral cavity, pharynx, and larynx. The most common types of assessments performed to aid in defining swallowing dysfunction are discussed below. These assessment tools are intended to help health care providers obtain information about swallow timing, pressure, and strength of the movements involved in the swallowing process.27,28
Modified Barium Swallow
The modified barium swallow, also called a videofluoroscopic swallowing study, is a radiologic examination of swallowing function that uses a special moving-type video X-ray called fluoroscopy. This test allows the examiner to view the oral cavity and pharyngeal components of swallowing. The test can be extended to view the esophagus. To perform the test, the patient is asked to chew and swallow thin liquids, pudding, and food that requires chewing. As the patient chews and swallows, the examiner observes the process to identify abnormalities in the swallowing process. The test enables clinicians to define components of the swallowing and muscle function used in performing in a normal vs an abnormal manner. This test can be conducted in about 15 minutes and there is little X-ray exposure.29 The modified barium swallow test is a commonly used initial test to evaluate esophageal dysphagia.30
Endoscopy
Fiberoptic endoscopic examination of swallowing (FEES) is a procedure designed to assess swallowing function through the use of a small tube placed through the nasal cavity into the pharynx to observe the pharynx before, during, and after swallowing colored foods and fluids of different consistencies. The food is observed as it travels over the back of the tongue into the pharynx and as it disappears into the esophagus. The test is useful in determining aspiration and whether any food is left in the pharynx after the swallow. One limitation of this test is its inability to visualize the oral and esophageal stages of swallowing. This test can be performed in a lab or an office, but can also be done at the bedside in settings such as long term care facilities.29 The FEES and the modified barium swallow tests are considered complementary tests in diagnosing mechanical oropharyngeal dysphagia.27
Manometry
During manometry, a thin flexible tube (catheter) with sensors is placed through the nose, down the pharynx, and into the esophagus.29 Manometry is an assessment technique used to determine pressures in the pharynx and/or esophagus throughout the course of the swallowing process. Pressure is very important during swallowing. If pressure is not present at any point in the swallowing process, food lingers at that position rather than being pushed through the gastrointestinal tract.29
Ultrasound
Ultrasound is an imaging method in which high-frequency sound waves produce images of body structures. When evaluating for dysphagia, this assessment method can be used only in the oral cavity.29 The use of ultrasound technology is helpful when evaluating oral function during the swallowing process,29 but it is limited in that it does not allow for assessment of dysphagia related to pharynx dysfunction.
Complications
Patients with both oropharyngeal and esophageal dysphagia are at increased risk for developing aspiration pneumonia with increased morbidity and mortality rates.25 Untreated dysphagia can contribute to chronic and even fatal disorders such as compromised nutritional status, dehydration, and insidious and significant weight loss over time.23,25
Treatment
Treatment for dysphagia must be individualized for each dysphagia sufferer. The treatment course must be carefully defined depending on the cause and nature of the dysphagia and must address the underlying cause of the disease. For example, for people suffering from mechanical obstruction or motility/ neuromuscular dysphagia caused by chronic GERD, medications to control the condition can help alleviate the symptoms.31
Dysphagia symptoms related to motility and neuromuscular disorders can be relieved by dilation of the constricted passages or the use of OnabotulinumtoxinA (Botox) shots in the lower part of the esophagus. Medications such as calcium channel blockers can be prescribed to help relax the esophagus.31
Individuals suffering from Parkinson’s disease usually respond well to modifying food consistency; posture changes and increased sensory input are important aspects of this process. Clinicians can teach the patient compensatory strategies and therapeutic swallowing techniques.32
Compensatory strategies allow patients to focus on implementing techniques that facilitate the safe intake of food and fluids.33 A postural adjustment, “chin down” or “chin tuck,” a strategy in which the patient tucks the chin to protect the airway during swallowing, is a possible intervention.32 This strategy might facilitate swallowing by decreasing the distance between the epiglottis and pharyngeal wall, thus protecting the airway. It is important to mention that no significant research has demonstrated or measured the impact of these interventions in the prevention of aspiration pneumonia.34
A randomized, controlled trial conducted in 2008 examined the effects of three strategies to combat aspiration for older adults with Parkinson’s disease and dementia. The strategies were the chin-down (or chin-tuck) posture approach with the use of thin liquids, the use of nectarlike thickened liquids with no posture change, and the use of honeylike thick liquids with no posture adjustment. Study participants were tested on all three interventions. Of the 711 participants, 49% aspirated when using any of the interventions and 25% did not aspirate when using any of the three interventions. Of the participants with Parkinson’s disease (without dementia), 39% aspirated with the use of any of the strategies, whereas one-half of the patients suffering from dementia (with or without Parkinson’s) aspirated. Study participants who did not aspirate in one of the three interventions (thus aspirating in one or two of the strategies tested) had significant success with thin liquids using the chin-down posture with thin liquids as compared with those who used nectar-thickened liquids (1% vs 2%; p <.05) or honey-thickened liquids with no posture change (1% vs 9%; p <.0001).35
Interventions such as diet modification, exercises to strengthen weak facial muscles, and compensatory strategies (such as alternate liquids and solids) are used as a primary course of treatment due to their noninvasive nature. Alternating solids and liquids serves to moisten the oral cavity and throat in efforts to move the bolus and clear food into the esophagus. A study that examined standard swallowing compensation strategies reported a 41% increase in the number of study participants who regained swallowing function within six months of treatment.36
If the individual’s swallowing abilities do not improve with SLP treatment and diet modifications, medical and/or surgical interventions must be considered. For instance, in patients with progressive diseases such as amyotrophic lateral sclerosis (ALS), enteral nutrition might be necessary. It’s important to note that while enteral feeding can prevent aspiration, there are risks associated with this modality that must be considered. Enteral feeding prevents aspiration while the food flows forward from the stomach or jejunum into the remainder of the gastrointestinal tract. A dysfunctional upper esophageal sphincter and/or lower esophageal sphincter can cause the formula to backflow, thus increasing the risk of aspiration.37 The use of enteral feeding might be beneficial in the management of dysphagia in elderly patients who are expected to recover the ability to swallow.38,39 A study conducted by Neumann reported a 67% increase in swallowing function following 15 weeks of SLP swallowing treatment and feeding tube removal.40 This is not to say that for individuals with persistent dysphagia, the use of enteral feeding is not warranted. The risk-benefit ratio of the intervention needs to be evaluated.38,39
Nutrition Considerations
As previously noted, many chronic conditions and neurological disorders predispose older adults to develop dysphagia; this can lead to a compromised nutritional status. The inability to swallow properly as well as fear of or discomfort with eating can lead to food avoidance, which can result in inadequate nutrient intake and dehydration.34,41
While age-related changes should not influence the normal adult’s ability to swallow, the presence of comorbidities such as Parkinson’s disease, Alzheimer’s disease, ALS and other neurological conditions, head and neck cancers, brain tumors, and COPD may lead to symptoms that affect all stages of swallowing.34 Health care providers, including RDs, should be alert to the possibility of swallowing difficulties in patients with any of these diagnoses.34
The National Dysphagia Diet
Among the interventions used to manage dysphagia in older adults, modification of food texture and altering liquid consistencies help improve safety while facilitating oral consumption to meet the individual’s nutritional needs. The National Dysphagia Diet Task force has standardized nutrition therapy for the management of patients with dysphagia. Through consensus, this group of RDs, speech language pathologists, and researchers developed the National Dysphagia Diet in the early 2000s and introduced it in 2002. The diet consists of three levels of solid foods and four levels of liquids. The levels of solid foods include dysphagia purée, dysphagia mechanically altered, and dysphagia advance. Fluids include thin, nectarlike, honeylike, and spoon-thick.42 Aspects of the National Dysphagia Diet can be summarized as follows:
Dysphagia Level I. The purée diet consists of foods that are puréed, homogenous, and cohesive. Foods should be prepared to the consistency of “pudding-like” products. Foods that require the individual to form a bolus, or controlled manipulation and chewing, are not permitted in this diet level. Foods with coarse textures such as raw fruits or vegetables and nuts are not permitted.42
This diet is intended for those with moderate to severe dysphagia, with reduced ability to protect their airway. Individuals on this diet often require close to total supervision during meals.42 Dysphagia Level I purée diets are commonly prescribed for patients at risk of choking with regular foods due to poor chewing ability, poor oral control, and weak pharyngeal constrictor movements.43 Intake for patients on Dysphagia Level I purée diets should be carefully monitored as this diet level is not always well accepted and patients tend to have a lower meal intake.34,44
Dysphagia Level II. The mechanically altered diet contains foods that are moist, soft-textured, and effortlessly shape into a bolus. This diet builds on the previous level diet; all foods on Dysphagia Level I are permitted with this diet. Meats and other select foods may be ground or minced into small pieces no larger than one-quarter of an inch. All food items must be easy to chew.42
Individuals with swallowing problems use the mechanically altered diet to facilitate chewing and moving the food around in the oral cavity. Its use decreases the possibility of food entering the trachea, thus safeguarding against aspiration.42
Dysphagia Level III. The advanced diet allows foods that are almost normal texture with the exception of crunchy, sticky, or very hard foods. Moist, bite-size foods are included in the diet. All foods included in Levels l and II are also allowed in this level, which is considered a transition to a regular diet. Individuals on this diet must have functional dentition and the ability to chew all the foods included in the diet, which includes fruits, vegetables, grains, meats and meat substitutes, and dairy foods.42 This diet is usually well tolerated by individuals with mild oropharyngeal dysphagia.
Liquids. Thickened liquids are widely used as a compensatory intervention in health care facilities and are also recommended for community-dwelling individuals who suffer from dysphagia. Thin liquids do not hold together in the oral cavity and can be easily aspirated into the lungs, increasing the risk of the development of aspiration pneumonia. The thicker the liquid, the lower the risk of aspiration. Clinical practice and anecdotal evidence support the use of thickened liquids as a way to help control how liquids move around the oral cavity. Thickened liquids are believed to decrease the pace and allow for better control of fluids during swallow.34
Some liquids are naturally thick; others are made so by the addition of commercial thickeners in the form of powders or gels. As previously stated, liquids for the treatment of dysphagia are classified as thin, nectar-like thick, honey-like thick, and spoon-thick. Thin regular liquids run quickly when poured from a spoon with very little to no coating left behind. They include items such as water, milk, juice, coffee, tea, carbonated drinks, and food items that are liquid at room temperature, such as gelatin or ice cream. Nectar-like liquids pour in a continuous stream from a spoon, leaving a thin coating behind. Liquids such as fruit nectars, maple syrup, eggnog, and tomato juice are naturally occurring nectar-like liquids. Honey-like liquids pour very slowly from a spoon and leave a heavy coating behind. Food items such as honey and thick cream soups are examples of naturally occurring honey-like thickened liquids. Spoon-thick liquids hold their shape when portioned with a spoon; pudding is an example.34,42
It is important to note concerns surrounding the overuse of thickened liquids. Current research does not clearly define the parameters for thickening that correlate to specific swallowing deficits nor specify those individuals who benefit from thickening.34 Patients often complain about the taste and mouthfeel of thickened liquids. This can affect the total volume of fluids consumed. The decrease in overall fluid intake, thus decreasing exposure to risk, can be the cause of the positive outcomes (reducing overall fluid intake is a positive outcome?) seen with the use of thickened liquids (vs the consistency itself).1 For patients on spoon-thick liquids, the total volume of liquids consumed can interfere with the overall meal intake, thus increasing the risk for compromised nutritional status. The feeling of satiety increases with the consumption of viscous fluids. In health care facilities, most meal offerings expose patients to a minimum of 1,200 mL of fluid per day. Spoon-thick liquids, which are thickened to a pudding consistency, must be eaten as part of the meal.
Consuming pudding-thick liquids competes with the intake for the meal. The same principle applies for community-dwelling individuals with dysphagia who must consume pudding-thick liquids as part of their dysphagia treatment.1 Overall, there is limited evidence supporting the use of thickened liquids to promote positive heath outcomes in relation to improvement in nutritional status or preventing aspiration pneumonia.34
The Dining Experience
For institutionalized patients, allowing for adjustments to the dining environment can help to foster better meal intake and independence in self-feeding. Aspects of dining such as presenting the meal, making the best use of the physical space available, using small tables (to allow for closer supervision and meal assistance), monitoring noise level, providing proper lighting, and manipulating seating arrangements to allow for the companionship of appropriate and similar peers can help to enhance the dining experience. Allowing real-time choice—enabling patients to select meals at point of service—can help to improve overall intake.34,45 Serving foods at the right temperature and rounding out the meal with the proper condiments and spices can help to enhance the meal experience.
Meals should be served in a timely manner, and sufficient time must be allocated for meal completion.
Research in developed countries indicates that 15% of community-residing older adults, 20% to 65% of hospitalized patients, and as many as 85% of patients in skilled nursing facilities and nursing homes experience malnutrition.46 Interventions that improve intake help dysphagia sufferers to sustain well-nourished bodies, which can help overcome the impaired muscle function that can occur as a comorbid condition of dysphagia. Offering nutrient-dense snacks and encouraging fluid consumption throughout the day can help promote adequate energy intake and reduce the risk of dehydration.47
Oral supplementation and consumption of nutrient-dense foods are common interventions for dysphagia patients who are trying to maintain their weight.38
Free Water Protocol
The Free Water Protocol program permits individuals on a thickened-liquids diet (or those having a nothing by mouth, or NPO, status) to enjoy ice chips and/or free water according to predetermined guidelines. Since the Free Water Protocol program is not appropriate for everyone, the SLP must evaluate all patients using exclusion criteria, which include an acute or unstable medical condition, coughing risk that can increase pain or discomfort, uncontrolled oral cavity infections, presence of poor oral hygiene even when routine oral care is provided, severe coughing with oral intake, and the presence of pneumonia.48
Individuals who meet inclusion criteria are allowed to consume water between meals and 30 minutes after each meal. Individuals must sit upright while consuming water and utilize any swallowing strategies that have been prescribed for them by the SLP.49 Routine oral care must be provided (swab mouth or rinse-and-spit to be performed prior to any water intake) to all program participants prior to consuming water.48 Research on the Free Water Protocol program indicates that good oral hygiene is key to the success of the program. Frequent oral care can help to reduce oral bacteria associated with aspiration pneumonia, on the grounds that any water entering the lungs seems to be reabsorbed into the bloodstream,50 although Feinberg reports that in most cases, aspiration of water results in no episodes of aspiration pneumonia.51 Research indicates that aspiration of thickened liquids and food is a significantly greater contributor to aspiration pneumonia than is water.50 A chart review conducted at the Frazier Rehab over an 18-month period reported that only two patients from the 234-patient sample developed aspiration pneumonia while on the Free Water Protocol. The patients who developed aspiration pneumonia were suspected of aspirating food.49
Implications for Practice
The aging process has an impact on the normal swallowing function and process. Older adults are at higher risk for developing dysphagia due to the fact that conditions that impact the swallowing function are more common in this age group. Dysphagia evaluation and management is a multidisciplinary team effort. The process requires health care providers to conduct a detailed history and identify the root cause by distinguishing between oropharyngeal and esophageal dysphagia, as well as differentiating mechanical obstruction from neuromuscular disorder.
RDs play a major role in identifying individuals with dysphagia. Timely identification of dysphagia symptoms is essential to ensure timely referrals to SLPs for dysphagia treatment and prompt nutrition interventions. The RD’s expert knowledge of crafting diets facilitates the development of an individualized meal program to target the individual’s symptoms and nutritional needs; the individual’s ability to consume food and fluids greatly contributes to overall physical and emotional well-being.
— Written by Nancy Munoz, DCN, MHA, RDN, LDN, FAND, a lecturer in the department of nutrition at the University of Massachusetts Amherst, an instructor for the University of Phoenix College of Nursing and Health Care program, and the assistant chief of nutrition and food services for the Southern Nevada Veterans Administration Healthcare System.
References
1. Crary MA, Groher ME. Introduction to Adult Swallowing Disorders. St. Louis, MO: Butterworth-Heinemann; 2003.
2. Ekberg O, Hamdy S, Woisard V, Wuttge-Hannig A, Ortega P. Social and psychosocial burden of dysphagia: its impact on diagnosis and treatment. Dysphagia. 2002;17(2):139-146.
3. Muder RR. Pneumonia in residents of long-term care facilities: epidemiology, etiology, management, and prevention. Am J Med. 1998;105(4):319-330.
4. Vergis EN, Brennen C, Wagener M, Muder RR. Pneumonia in long-term care: a prospective case-control study of risk factors and impact on survival. Arch Intern Med. 2001;161(19):2378-2381.
5. Serra-Prat M, Palomera M, Gomez C, et al. Oropharyngeal dysphagia as a risk factor for malnutrition and lower respiratory tract infection in independently living older persons: a population-based prospective study. Age Ageing. 2012;41(3):376-381.
6. Marik PE, Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124(1):328-336.
7. Barczi SR, Sullivan PA, Robbins J. How should dysphagia care of older adults differ? Establishing optimal practice patterns. Semin Speech Lang. 2000;21(4):347-361.
8. Wilkins T, Gillies RA, Thomas AM, Wagner PJ. The prevalence of dysphagia in primary care patients: a HamesNet Research Network study. J Am Board Fam Med. 2007;20(2):144-150.
9. Barer DH. The natural history and functional consequences of dysphagia after hemispheric stroke. J Neurol Neurosurg Psychiatry. 1989;52(2):236-241.
10. Gordon C, Hewer RL, Wade DT. Dysphagia in acute stroke. Br Med J (Clin Res Ed). 1987;295(6595):411-414.
11. Mann G, Hankey GJ, Cameron D. Swallowing function after stroke: prognosis and prognostic factors at 6 months. Stroke. 1999;30(4):744-748.
12. Calcagno P, Ruoppolo G, Grasso MG, De Vincentiis M, Paolucci S. Dysphagia in multiple sclerosis — prevalence and prognostic factors. Acta Neurol Scand. 2002;105(1):40-43.
13. De Pauw A, Dejaeger E, D’hooghe B, Carton H. Dysphagia in multiple sclerosis. Clin Neurol Neurosurg. 2002;104(4):345-351.
14. Coates C, Bakheit AM. Dysphagia in Parkinson’s disease. Eur Neurol. 1997;38(1):49-52.
15. Locke GR 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ 3rd. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112(5):1448-1456.
16. Siebens H, Trupe E, Siebens A, et al. Correlates and consequences of eating dependency in institutionalized elderly. J Am Geriatr Soc. 1986;34(3):192-198.
17. Robbins J, Bridges AD, Taylor A. Oral, pharyngeal and esophageal motor function in aging. GI Motility Online website. http://www.nature.com/gimo/contents/pt1/full/gimo39.html. Published May 16, 2006.
18. Swallowing. MedlinePlus website. http://www.nlm.nih.gov/medlineplus/ency/anatomyvideos/000126.htm. Updated November 18, 2015.
19. DiMarino MC. Dysphagia. Merck Manual: Professional Version website. http://www.merckmanuals.com/professional/gastrointestinal_disorders/esophageal_and_swallowing_disorders/dysphagia.html. Updated May 2014. Accessed August 1, 2015.
20. Bernstein M, Munoz N. Nutrition for the Older Adult. 2nd ed. Burlington, MA: Jones & Bartlett Learning; 2014.
21. Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. J Speech Lang Hear Res. 2000;43(5):1264-1274.
22. Logemann JA, Pauloski BR, Rademaker AW, Kahrilas PJ. Oropharyngeal swallow in younger and older women: videofluoroscopic analysis. J Speech Lang Hear Res. 2002;45(3):434-445.
23. Loue S, Sajatovic M, eds. Encyclopedia of Aging and Public Health. New York, NY: Springer Science+Business Media; 2008.
24. Spieker MR. Evaluating dysphagia. Am Fam Physician. 2000;61(12):3639-3648.
25. Garon BR, Sierzant T, Ormiston C. Silent aspiration: results of 2,000 video fluoroscopic evaluations. J Neurosci Nurs. 2009;41(4):178-185.
26. McCulloch TM, Jaffe D. Head and neck disorders affecting swallowing. GI Motility Online website. http://www.nature.com/gimo/contents/pt1/full/gimo36.html. Published May 16, 2006.
27. Aslam M, Vaezi MF. Dysphagia in the elderly. Gastroenterol Hepatol (NY). 2013;9(12):784-795.
28. Cook IJ, Dodds WJ, Dantas RO, et al. Timing of videofluoroscopic, manometric events, and bolus transit during the oral and pharyngeal phases of swallowing. Dysphagia. 1989;4(1):8-15.
29. Fass R. Overview of dysphagia in adults. UpToDate website. http://www.uptodate.com/contents/overview-of-dysphagia-in-adults. Updated January 8, 2014.
30. Martin-Harris B, Jones B. The videofluorographic swallowing study. Phys Med Rehabil Clin N Am. 2008;19(4):769-785, viiii.
31. Dysphagia. Mayo Clinic website. http://www.mayoclinic.org/diseases-conditions/dysphagia/basics/definition/con-20033444. Updated October 15, 2014. Accessed August 1, 2015.
32. Tjaden K. Speech and swallowing in Parkinson’s disease. Top Geriatr Rehabil. 2008;24(2):115-126.
33. Groher ME, Crary MA. Dysphagia: Clinical Management in Adults and Children. Maryland Heights, MO: Mosby Elsevier; 2010.
34. Sura L, Madhavan A, Carnaby G, Crary MA. Dysphagia in the elderly: management and nutritional considerations. Clin Interv Aging. 2012;7:287-298.
35. Logemann JA, Gensler G, Robbins J, et al. A randomized study of three interventions for aspiration of thin liquids in patients with dementia or Parkinson's disease. J Speech Lang Hear Res. 2008;51(1):173-183.
36. Carnaby G, Hankey GJ, Pizzi J. Behavioural intervention for dysphagia in acute stroke: a randomised controlled trial. Lancet Neurol. 2006;5(1):31-37.
37. DeMeo MT, Bruninga K. Physiology of the aerodigestive system and aberrations in that system resulting in aspiration. JPEN J Parenter Enteral Nutr. 2002;26(6 Suppl):S9-S17; discussion S17-S18.
38. Janssens JP. Pneumonia in the elderly (geriatric) population. Curr Opin Pulm Med. 2005;11(3):226-230.
39. Metheny NA. Preventing aspiration in older adults with dysphagia. Try This: Best Practices in Nursing to Care for Older Adults website. http://consultgerirn.org/uploads/File/trythis/try_this_20.pdf. Updated 2012. Accessed August 1, 2015.
40. Neumann S. Swallowing therapy with neurologic patients: results of direct and indirect therapy methods in 66 patients suffering from neurological disorders. Dysphagia. 1993;8(2):150-153.
41. Logemann JA. Evaluation and Treatment of Swallowing Disorders. 2nd ed. Austin, TX: Pro ed; 1998.
42. Nutrition Care Manual products. Nutrition Care Manual website. https://www.nutritioncaremanual.org/. Accessed June 12, 2015.
43. Perlman PW, Cohen MA, Setzen M, et al. The risk of aspiration of pureed food as determined by flexible endoscopic evaluation of swallowing with sensory testing. Otolaryngol Head Neck Surg. 2004;130(1):80-83.
44. Hotaling DL. Nutritional considerations for the pureed diet texture in dysphagic elderly. Dysphagia. 1992;7(2):81-85.
45. Hotaling DL. Adapting the mealtime environment: setting the stage for eating. Dysphagia. 1990;5(2):77-83.
46. Morley JE. Anorexia of aging: physiologic and pathologic. Am J Clin Nutr. 1997;66(4):760-773.
47. Wright L, Cotter D, Hickson M. The effectiveness of targeted feeding assistance to improve the nutritional intake of elderly dysphagic patients in hospital. J Hum Nutr Diet. 2008;21(6):555-562.
48. Carlaw C, Finlayson H, Beggs K, et al. Outcomes of a pilot water protocol project in a rehabilitation setting. Dysphagia. 2012;27(3):297-306.
49. Panther K. The Frazier Free Water Protocol. SIG 13 Perspect Swallowing Swallowing Disord (Dysphagia). 2005;14:4-9.
50. Holas MA, DePippo KL, Reding MJ. Aspiration and relative risk of medical complications following stroke. Arch Neurol. 1994;51(10):1051-1053.
51. Feinberg MJ, Knebl J, Tully J, Segall L. Aspiration and the elderly. Dysphagia. 1990;5(2):61-71.