This course appeared as the CPE Monthly in the May 2015 issue of Today's Dietitian. For a printable PDF of this course and exam, click here.
The American Cancer Society estimates that 54,870 new cases of endometrial (uterine) cancer will be diagnosed in 2015, with 10,170 deaths resulting.1 Compared with 2010 estimates, this represents a 26% increase in new cases and a 22% increase in deaths. Most cases of endometrial cancer are diagnosed in women older than 55 (mean age of 61.2 at diagnosis),2 and the lifetime risk of developing this type of cancer is one in 37.1
It’s estimated that there are more than 600,000 endometrial cancer survivors in the United States,1 a number that surely will climb if incidence continues to rise. Effective lifestyle interventions, including medical nutrition therapy (MNT) that RDs provide, are needed to help reduce the risk of this type of cancer and treat the rapidly increasing number of newly diagnosed women and survivors.
Types and Risk Factors
Endometrial cancer, which starts in the cells lining the uterus, is the most common cancer of the female reproductive organs in the United States.1
More than 80% of endometrial cancers are adenocarcinomas, or cancers of the cells that form glands in the endometrium. These are classified as type 1 endometrial cancers because they’re thought to be caused by excess estrogen. Other less common forms of endometrial cancer include clear-cell carcinoma, serous carcinoma, and poorly differentiated carcinoma. These are called type 2 endometrial cancers because they’re more likely to spread outside the uterus.
Uterine carcinosarcoma is another type of endometrial cancer with features of both endometrial carcinoma and sarcoma. These uterine sarcomas, which start in the muscle walls of the uterus, are less common than endometrial cancer and different in terms of treatment and prognosis.
The American Cancer Society has identified the following factors that increase endometrial cancer risk:1
- medical conditions such as obesity, diabetes, polycystic ovarian syndrome, and endometrial hyperplasia;
- medications such as estrogen replacement therapy that’s given alone without progesterone or tamoxifen;
- obstetric conditions such as nulliparity (not giving birth), early menarche, late menopause, and infrequent menses;
- medical history of previous breast or ovarian cancer diagnosis or prior pelvic radiation;
- family history of Lynch syndrome (a hereditary condition that increases the risk of colon cancer); and
- lifestyle factors such as a high-fat diet and lack of physical activity.
Endometrial cancer can be four times more common in women with diabetes, even if they aren’t overweight.1 The National Cancer Institute’s PDQ (Physician’s Data Query) cancer information summary about endometrial cancer risk factors identifies combination oral contraceptive agents and physical activity as protecting against this type of cancer. The use of oral contraceptives for four years reduced endometrial cancer risk by 56%, for eight years by 67%, and for 12 years by 72%.2 Self-reports of regular exercise have been associated with an estimated 38% to 46% decrease in endometrial cancer risk,2 but there’s no specific evidence regarding the intensity or duration of activity required to achieve this reduction.
Obesity is the most significant risk factor for endometrial cancer. Reeves and colleagues studied 86,937 women enrolled in the Women’s Health Initiative to determine the effects of BMI and waist-to-hip ratio on the incidence, stage, and grade of endometrial cancer. In a mean of 7.8 years of follow-up, women who were obese (BMI of 30 or higher) at baseline had a 76% higher endometrial cancer risk, and women with a waist-to-hip ratio of 0.853 or higher had a 33% higher risk.3 There was no association between BMI or waist-to-hip ratio with regard to cancer stage or grade. This is consistent with the results of several other studies that have shown a higher risk of endometrial cancer in obese women.4,5
The 2013 report Food, Nutrition, Physical Activity, and the Prevention of Endometrial Cancer indicated that the scientific evidence shows that body fatness, as measured by BMI, waist circumference, and adult weight gain, causes endometrial cancer. The scientific evidence also shows that increased glycemic load probably increases this cancer risk, and that physical activity and coffee (both with and without caffeine) protect against it. The evidence analysis, which previously identified red meat consumption as a risk factor for endometrial cancer and nonstarchy vegetable intake as protective against it, was found to be too inconsistent and weak in this new report.6
A recent study of the consumption of sugar-sweetened beverages, fruit juices, sugar-free beverages, sweets/baked goods, starches, and sugars and the risk of endometrial cancer in 23,039 postmenopausal women enrolled in the Iowa Women’s Health Study found that the risk of type 1 endometrial cancer was 78% higher in women with the highest intake of sugar-sweetened beverages and total sugar. This association was present regardless of BMI, physical activity, history of diabetes, or cigarette smoking. No association was found between these dietary components and type 2 endometrial cancer. This study has many strengths, including the large sample size and a complete analysis of all possible confounders, but further research is needed to confirm these findings.7
Secondary analysis of data from the VITamins and Lifestyle Cohort examined whether intakes of long-chain omega-3 fatty acids, including EPA and DHA from diet and supplements and fish, were associated with increased risk of endometrial cancer.8 Of 22,494 women enrolled, 263 cases were identified after nine years of follow-up. Dietary intake data was collected using the food frequency questionnaire used in the Women’s Health Initiative Study. Women with the highest intakes of dietary EPA and DHA had a 79% higher incidence of endometrial cancer compared with women with lower intakes. Women who had the highest fish consumption had more than a twofold increase in the risk of endometrial cancer. When the data were stratified by BMI (less than 25 vs 25 or greater), increases in risk were seen only in overweight and obese women, and significant reductions in risk were found in normal-weight women. These findings suggest the need for randomized trials to fully understand these associations.
The most current research investigating dietary factors in endometrial cancer used a Nutrient-wide Association Study to investigate 84 foods or nutrients in the European Prospective Investigation into Cancer and Nutrition (EPIC) study and the Nurses’ Health Study (NHS) and NHSII study.9 In 2,834 cases of endometrial cancer identified, 1,303 were from EPIC (mean follow-up was 11 years) and 1,531 were from NHS/NHSII (mean follow-up was 25 years).9 Dietary intake was collected using validated questionnaires or food records in EPIC and repeated food frequency questionnaires in NHS/NHSII. Higher intakes of coffee were associated with decreased risk while higher butter intake was associated with increased risk in all three cohorts. In the EPIC cases, 10 dietary factors were identified for which the highest vs lowest consumption was associated with increased risk (butter, yogurt, potatoes, and carbohydrates) or decreased risk (cheese, coffee, cream desserts, total fat, monounsaturated fat, and phosphorus). None of these findings was confirmed in the NHS/NHSII cohorts. This study was the first to find an association with butter intake and confirms previous findings about coffee intake. Again, more research is needed to confirm the mechanisms linking coffee and butter intake to endometrial cancer.
Diagnosis, Staging, and Treatment
Signs and symptoms of endometrial cancer typically occur early in the progression of the disease and are obvious, which is different from other gynecological cancers, such as ovarian cancer. The signs and symptoms include abnormal vaginal bleeding; thin, clear vaginal discharge after menopause; lower abdominal or pelvic pain or cramping; a pelvic mass; and unintentional weight loss. Almost 90% of women diagnosed with endometrial cancer experience abnormal vaginal bleeding.1
There’s no screening test or procedure for endometrial cancer, although an interventional trial at St Luke’s-Roosevelt Hospital in New York is recruiting 300 overweight or obese women with the goal of developing an endometrial biopsy-screening program.10 A gynecologist or gynecologic oncologist usually diagnoses endometrial cancer after an endometrial biopsy or dilation and curettage and examination of the endometrium tissue, though imaging tests such as transvaginal ultrasound, CT, cystoscopy, proctoscopy, and MRI also can be used to diagnosis this cancer.
Endometrial cancer is graded on a scale of 1 to 3. Grade 1 tumors have mostly normal-looking cells; grade 2 tumors have more abnormal-looking cells; and grade 3 tumors have the highest percentage of abnormal-looking cells. Grade 3 endometrial cancer is more likely to spread to other tissues.
If the cancer is grade 2 or 3, cancer antigen 125 (CA-125) levels may be measured, as blood levels of CA-125 are elevated as a result of ovarian, endometrial, peritoneal, and fallopian tube cancers. High levels would indicate that the cancer probably has spread beyond the uterus.
The primary treatment for endometrial cancer is a total hysterectomy performed either abdominally, vaginally, or laparoscopically. The uterus and cervix are removed, and lymph nodes in the pelvis and abdomen also may be removed for examination. A radical hysterectomy is a more extensive surgery that involves removing the uterus, cervix, and upper part of the vagina; this is performed if there are indications that the cancer has spread. A bilateral salpingo-oophorectomy (removal of both fallopian tubes and ovaries) also is a common procedure in such cases.
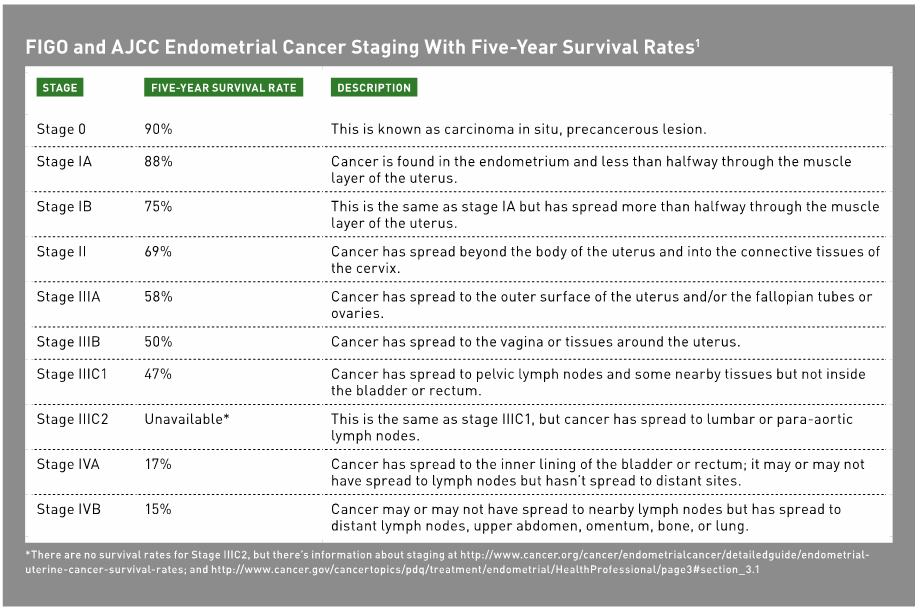
Staging of endometrial cancer occurs during or after surgery, when complete information about all cancerous tissues has been obtained. Two different staging systems are used: one from the International Federation of Gynecology and Obstetrics (FIGO) and one from the American Joint Commission on Cancer (AJCC). They’re similar except that the FIGO system doesn’t have a stage 0.
Table 1 shows the AJCC and FIGO stages of endometrial cancer along with the five-year survival rates for each stage. It’s important to note that close to 68% of all endometrial cancers are diagnosed at stages IA, IB, or II, when survival rates are high.11
Adjuvant therapies for endometrial cancer are indicated based on the cancer stage. Radiation therapy typically is given in stages I (IA and IB) and II and can be administered by two means: vaginal cuff brachytherapy, which places radioactive material inside the vagina, or external-beam radiation therapy to the pelvis. In some cases, both therapies are given.
Chemotherapy is used after surgery and radiation for both stage III and IV cancers. The most common chemotherapeutic drug combinations are carboplatin with paclitaxel and cisplatin with doxorubicin. Chemotherapy can be given orally, through an IV, or interperitoneally. Progestin therapy with medications such as medroxyprogesterone (Provera) and megesterol (Megace) also can be used to stop cancer growth. Tamoxifen is used for advanced or recurrent cases of endometrial cancer.
Survivors’ Profiles
After active treatment for their cancers has ended, many endometrial cancer patients have reported that they aren’t prepared for entering the posttreatment phase of survivorship.12 Concerns include the risk of cancer recurrence; other health issues such as hypertension, type 2 diabetes, and cardiovascular disease; and long-term effects of the cancer.
Health care professionals need to address this gap in preparation because endometrial cancer survivors experience many health problems. A 2011 study of 120 stage I and II endometrial cancer survivors with a BMI of at least 25 found that 93% had abdominal obesity as measured by waist circumference, 43% had hypertension, 35% had osteoarthritis, 33% had metabolic syndrome, and 21% had type 2 diabetes.13
More than 70% of endometrial cancer patients were found to be overweight or obese in a retrospective study of 380 patients with early-stage endometrial cancer. This is higher than what’s found in the general population. This study showed that obesity increased the risk of mortality from all causes but not from endometrial cancer or cancer recurrence.14
However, a study by Calle and colleagues found that endometrial cancer survivors with a BMI higher than 40 had a 6.25 relative risk of death, which is the highest observed relative risk of death for any obesity-driven cancer.15 Molecular mechanisms such as chronic inflammation, hyperinsulinemia, and insulin resistance that may explain the association between obesity and endometrial cancer have been explored,16 but more research is needed.
Obesity is, in part, responsible for the diagnosis of endometrial cancer. However, survivors die at higher rates from conditions associated with obesity, rather than their cancer diagnoses. Their obesity also affects their quality of life, as shown in a prospective study of 152 stage I and II endometrial cancer survivors. These women completed the short-form medical outcomes survey (SF-36) and a cancer-specific quality-of-life survey on the functional assessment of cancer therapy (FACT-G). Lower quality-of-life scores were noted in several domains of both surveys in obese and morbidly obese endometrial cancer survivors when compared with normal and overweight survivors.17
Physical Activity
The American College of Sports Medicine (ACSM) published exercise recommendations for cancer survivors in 2010 and confirmed that exercise is safe both during and after cancer treatment. The overall recommendation is that cancer survivors, except when contraindicated, should follow the US Department of Health and Human Services 2008 Physical Activity Guidelines for Americans, which call for 150 minutes of moderate or 75 minutes of vigorous physical activity per week for adults aged 18 to 64. The American Cancer Society issued an update of its 2006 Nutrition and Physical Activity for Cancer Survivors recommendation in 2012, and its recommendations supported those in the ACSM 2010 report.
Physical activity may be especially beneficial for endometrial cancer survivors given their obesity and comorbidities. However, there has been little research specifically on physical activity in this population. A 2010 case-control study by Friedenreich and colleagues, conducted in Alberta, Canada, compared lifetime total physical activity in 542 cases and 1,032 controls to determine differences in endometrial cancer risk. They found that risk was significantly reduced with greater recreational activity, but not household or occupational activity, and risk increased with sedentary behavior.18
There has been recent interest in studying the impact of self-efficacy in adhering to exercise recommendations. Hughes and colleagues compared the effects of a single exercise session on self-efficacy in 20 endometrial cancer survivors and 19 controls using a submaximal cycle ergometry test. Participants were placed on a metabolic cart to measure oxygen and carbon dioxide exchange and were asked to pedal on the stationary bike, with increases in resistance every six seconds until reaching 85% of predicted maximum heart rate, a respiratory exchange ratio greater than 1, or signaling a desire to stop. Results showed the endometrial cancer survivors had greater self-efficacy compared with controls, and they were more likely to engage in subsequent exercise sessions.19
Steps to Study Health, a study funded by the National Institutes of Health, examined the long-term effects of a six-month behavioral intervention using social cognitive theory—a well-researched theory of how people adopt new behaviors. Self-efficacy and outcome expectations about physical activity, as well as exercise duration, were measured in stage I, II, and IIIA endometrial cancer survivors. Participants’ mean age was 57 (±11.01), and mean BMI was 34.2 (±9.4), and 80% of the women had stage I cancer. Seventy-five women completed all measurements, which included seven days of home-based physical activity measurements and one 2- to 3-hour physical activity assessment in an exercise physiology laboratory. Results showed that the women performed more minutes of exercise on the days when their morning levels of self-efficacy and positive outcome expectations were higher.20
The authors of this study suggest that endometrial cancer survivors may require unique interventions to improve exercise adherence although more studies with larger sample sizes are needed.
MNT
There’s no evidence that MNT will reduce recurrence of or mortality from endometrial cancer. There are two distinct opportunities for providing MNT for women diagnosed with endometrial cancer: during treatment and during the first few years of survivorship. Because surgery is the primary treatment for endometrial cancer, women will be hospitalized for at least 24 hours and should be screened for nutritional problems such as involuntary weight loss, iron deficiency due to abnormal bleeding before diagnosis, and changes in appetite or digestion postsurgery. Protein and calories should be provided to meet individual requirements for healing from surgery per the Evidence Analysis Library Guidelines for Oncology.
The side effects of adjuvant treatments for endometrial cancer can have serious implications for dietary intake, a patient’s quality of life, and ability to return to baseline activities. Common side effects of radiation therapy for endometrial cancer include diarrhea, fatigue, red and painful skin at the radiation site, radiation cystitis, radiation proctitis, radiation vaginitis, vaginal stenosis, and lymphedema. Most patients receive some level of radiation, so awareness of these side effects is important for dietetics professionals.
Chemotherapy for advanced cases can cause side effects such as nausea, vomiting, anorexia, mouth sores, low white and red blood cell and platelet counts, peripheral neuropathy, and hemorrhagic cystitis.1 MNT for these side effects is the same for endometrial cancer patients as it is for patients with other types of cancer. RDs should monitor patients’ weight and hydration status; provide education about the importance of food safety; recommend smaller, more frequent meals that include nutrient-dense foods; and help patients identify resources for assistance with shopping and cooking.
Usual care after endometrial cancer treatment includes annual visits with a gynecologic oncologist for five years. As previously noted, many endometrial cancer survivors report that they feel they aren’t as adequately prepared for survivorship as are other cancer survivors, such as those who have had breast cancer. RDs can advocate for changes in usual care for endometrial cancer survivors.
As the incidence and mortality from endometrial cancer increases, interventions need to be planned and implemented to prevent this cancer and reduce mortality by targeting overweight and obesity.
There are only three published lifestyle intervention studies conducted in the United States that have targeted endometrial cancer survivors. In a pilot randomized controlled trial by von Grueningen and colleagues, 45 overweight or obese stage I and II endometrial cancer survivors were randomized to a six-month multidisciplinary lifestyle intervention or usual care. The primary outcome was weight change, and secondary outcomes were changes in physical activity and dietary intake.21
At 12 months, the lifestyle intervention group lost 3.5 kg (roughly 7.7 lbs) compared with a 1.4-kg gain (roughly 3 lbs) in the control group and had significantly increased physical activity. The results demonstrated feasibility and effectiveness of a lifestyle intervention in endometrial cancer survivors.21
The same research group refined its methods and expanded the lifestyle intervention in the Survivors of Uterine Cancer Empowered by Exercise and Healthy Diet (SUCCEED) trial. In this study, 75 overweight or obese endometrial cancer survivors were randomized either to a lifestyle intervention or a usual care group. The primary research outcome was to determine whether the lifestyle intervention resulted in greater weight change at 12 months compared with the usual care group.
Participants in the usual care group received an informational brochure about the benefits of and strategies for weight loss.22 The lifestyle intervention lasted six months, with 10 weekly sessions followed by six biweekly sessions. Monthly contact by the RD with each participant in the intervention group via phone or newsletter occurred for six months after the intervention ended.22
A multidisciplinary team planned and delivered the intervention, including an RD, who provided content for every session as well as private weigh-ins with individual feedback about progress and goals; a physical therapist, who led two sessions on resistance training; and a clinical psychologist, who led three sessions on behavior change. There was no specific diet prescribed as far as calorie intake or macronutrient distribution. Nutrition targets for the intervention were centered on the MyPyramid food groups’ recommendations and choosing nutrient-dense foods.22
The RD also delivered the physical activity portion of the intervention, which had been planned by a well-known researcher in the field of physical activity for cancer survivors. The target for physical activity was 150 minutes of moderate activity or 75 minutes of vigorous activity per week and 20 minutes of resistance training three days per week. The participants were given pedometers, heart rate monitors, ankle weights, and water bottles.
The mean BMI and waist circumference of the two groups was 36.4 (±5.5) and 42.1 (±4.9) inches for the intervention group, and 36.5 (±9.6) and 41.6 (±5.9) inches for the usual care group. Thirty-two percent of the women had a BMI of 40 or higher, and 33% had hypertension, 27% had osteoarthritis, and 21% had diabetes.
The results of this study showed that exercise behavior change and weight loss were achieved in this population of cancer survivors. The mean difference in weight change between the intervention and usual care groups at six months was -4.4 kg (roughly 9.7 lbs) and at 12 months was -4.6 kg (roughly 10.1 lbs). The mean difference in minutes of physical activity between the groups at six months was 100 and at 12 months was 89 ([14, 163], p = 0.020). Thirty percent of the women in the lifestyle intervention met the weight-loss target of 5% of baseline weight.22
In a substudy of the SUCCEED trial, Nock and colleagues compared neural activation with high-calorie food cues during both fed and fasted states in eight participants in the lifestyle intervention group using functional MRI. The women were studied both before and after the six-month intervention. The results showed that the women had decreased neural activation to high-calorie food cues after the intervention, suggesting that lifestyle interventions that target weight loss may reduce high-calorie food rewards in obese endometrial cancer survivors.23
An expansion of the SUCCEED study, Revving Up Exercise for Sustained Weight Loss by Altering Neurological Reward and Drive (REWARD), is recruiting obese stage I endometrial cancer survivors for weight-loss intervention with assisted exercise on a stationary bicycle, functional MRI, and genetic sampling.24 The purpose of REWARD is to examine whether assisted exercise leads to weight loss, improves quality of life, increases motivation to exercise, and causes changes in food behaviors as measured by neuronal changes in response to high- and low-calorie food cues. It’s important for dietetics professionals to watch for the results of this study and other studies so they can provide effective interventions for this high-risk population.
Summary and Conclusions
While there’s no scientific evidence that weight loss will decrease endometrial cancer recurrence or mortality, there’s research showing that endometrial cancer survivors have higher all-cause mortality, especially with BMIs of 40 or higher, and that their obesity compromises their quality of life.
Given the serious health risks in this population of cancer survivors and the fact that the number of endometrial cancer cases is increasing, it’s imperative that RDs take action to provide MNT with an emphasis on weight loss and treatment of these patients’ multiple comorbidities.
There are many actions RDs can and should take to ensure that all women are informed about the risk factors for endometrial cancer. Dietitians should closely monitor all overweight and obese patients, especially those who are postmenopausal, to ensure they’re educated about their risk of developing endometrial cancer. When screening procedures for endometrial cancer are validated and incorporated into standard care, dietitians should ensure that their overweight and obese postmenopausal patients are screened.
Specific recommendations to decrease the risk of developing endometrial cancer should be provided to women of all ages and should emphasize achieving and maintaining a healthful body weight, participating in daily physical activity, and avoiding sugar-sweetened beverages.
RDs are uniquely qualified to collaborate with other health care practitioners in cancer centers and clinics to plan and implement multidisciplinary interventions for endometrial cancer survivors. A diagnosis of endometrial cancer should become a teachable moment for making significant changes to diet and physical activity. Patients often aren’t aware of their increased risk of morbidity and mortality from causes other than their cancer, and this information can help motivate them to improve their weight status. The RD’s expertise is essential to high-quality interventions that promote weight loss and physical activity while ensuring that comorbid conditions such as hypertension, diabetes, and hyperlipidemia that are common in this population of cancer survivors are treated appropriately.
Finally, it’s critical that RDs follow research that identifies molecular mechanisms that explain the relationship between obesity and endometrial cancer and that clarifies the relationship between sugar-sweetened beverage intake and endometrial cancer. They also should follow and hopefully conduct research to see if weight loss interventions in this population impact survival.
— Written by Mary Beth Kavanagh, MS, RDN, LD, a senior instructor in the department of nutrition at Case Western Reserve University in Cleveland who has been conducting research on endometrial cancer and weight management since 2005.
References
1. Endometrial (uterine) cancer. American Cancer Society website. http://www.cancer.org/acs/groups/cid/documents/webcontent/003097-pdf.pdf. Last revised February 3, 2015. Accessed March 17, 2015
2. General information about endometrial cancer. National Cancer Institute website. http://www.cancer.gov/cancertopics/pdq/treatment/endometrial/HealthProfessional/page1. Accessed May 27, 2014.
3. Reeves KW, Carter GC, Rodabough RJ, et al. Obesity in relation to endometrial cancer risk and disease characteristics in the Women’s Health Initiative. Gynecol Oncol. 2011;121(2):376-382.
4. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569-578.
5. Friedenreich C, Cust A, Lahmann PH, et al. Anthropometric factors and risk of endometrial cancer: the European prospective investigation into cancer and nutrition. Cancer Causes Control. 2007;18(4):399-413.
6. World Cancer Research Fund/American Institute for Cancer Research. Endometrial Cancer 2013 Report: Food, Nutrition, Physical Activity, and the Prevention of Endometrial Cancer. https://files.acrobat.com/a/preview/6b636595-21f9-4fb2-8b13-7e634f5802e5. Published 2013. Accessed May 29, 2014.
7. Inoue-Choi M, Robien K, Mariani A, Cerhan JR, Anderson KE. Sugar-sweetened beverage intake and the risk of type I and type II endometrial cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2013;22(12):2384-2394.
8. Brasky TM, Neuhouser ML, Cohn DE, White E. Associations of long-chain ω-3 fatty acids and fish intake with endometrial cancer risk in the VITamins And Lifestyle cohort. Am J Clin Nutr. 2014;99(3):599-608.
9. Merritt MA, Tzoulaki I, Tworoger SS, et al. Investigation of dietary factors and endometrial cancer risk using a nutrient-wide association study approach in the EPIC and Nurses’ Health Study (NHS) and NHSII. Cancer Epidemiol Biomarkers Prev. 2015;24(2):466-471.
10. St Luke’s Roosevelt Hospital Center. Screening for endometrial abnormalities in overweight and obese women. In: ClinicalTrials.gov. Bethesda, MD: National Library of Medicine. http://clinicaltrials.gov/ct2/show/NCT01922778: NCT01922778. Accessed May 28, 2014.
11. SEER stat fact sheets: endometrial cancer. National Cancer Institute website. http://seer.cancer.gov/statfacts/html/corp.html. Accessed January 31, 2014.
12. Jones JM, Ferguson S, Edwards E, Walton T, McCurdy N, Howell D. Experiences of care delivery: endometrial cancer survivors at end of treatment. Gynecol Oncol. 2012:124(3):458-464.
13. von Gruenigen VE, Waggoner SE, Frasure HE, et al. Lifestyle challenges in endometrial cancer survivorship. Obstet Gynecol. 2011;117(1):93-100.
14. von Gruenigen VE, Tian C, Frasure H, Waggoner S, Keys H, Barakat RR. Treatment effects, disease recurrence, and survival in obese women with early endometrial carcinoma: a Gynecologic Oncology Group study. Cancer. 2006;107(12):2786-2791.
15. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med. 2003;348(17):1625-1638
16. Schmandt RE, Iglesias DA, Co NN, Lu KH. Understanding obesity and endometrial cancer risk: opportunities for prevention. Am J Obstet Gynecol. 2011;205(6):518-525.
17. Fader AN, Frasure HE, Gil KM, Berger NA, von Grueningen VE. Quality of life in endometrial cancer survivors: what does obesity have to do with it? Obstet Gynecol Int. 2011;2011:308609. doi: 10.1155/2011/308609.
18. Friedenreich CM, Cook LS, Magliocco AM, Duggan MA, Courneya KS. Case-control study of lifetime total physical activity and endometrial cancer risk. Cancer Causes Control. 2010;21(7):1105-1116.
19. Hughes D, Baum G, Jovanovic J, Carmack C, Greisinger A, Basen-Engquist K. An acute exercise session increases self-efficacy in sedentary endometrial cancer survivors and controls. J Phys Act Health. 2010;7(6):784-793.
20. Basen-Engquist K, Carmack CL, Li Y, et al. Social-cognitive theory predictors of exercise behavior in endometrial cancer survivors. Health Psychol. 2013;32(11):1137-1148.
21. von Gruenigen VE, Courneya KS, Gibbons HE, Kavanagh MB, Waggoner SE, Lerner E. Feasibility and effectiveness of a lifestyle intervention program in obese endometrial cancer patients: a randomized trial. Gynecol Oncol. 2008;109(1):19-26.
22. von Gruenigen V, Frasure H, Kavanagh MB, et al. Survivors of uterine cancer empowered by exercise and healthy diet (SUCCEED): a randomized controlled trial. Gynecol Oncol. 2012;125(3):699-704.
23. Nock NL, Dimitropolous A, Tkach J, Frasure H, von Gruenigen V. Reduction in neural activation to high-calorie food cues in obese endometrial cancer survivors after a behavioral lifestyle intervention: a pilot study. BMC Neurosci. 2012;13:74.
24. Case Comprehensive Cancer Center. Assisted Exercise in Obese Endometrial Cancer Patients. In: ClinicalTrials.gov. Bethesda, MD: National Library of Medicine. http://clinicaltrials.gov/ct2/show/NCT01870947. Accessed February 8, 2014.